Introduction
The filum terminale (FT) is a fibrous band that extends from the conus medullaris to the periosteum of the coccyx, and its functions are to fixate, stabilize, and buffer the distal spinal cord from normal and abnormal cephalic and caudal traction. The cranial intradural part is called the FT internum, and the caudal extradural part is the FT externum [1,2]. The FT is composed largely of loose collagen fibers, small blood vessels, and occasional small nerve fascicles, and contains an extension of the central canal that is lined with ciliated ependymal cells [3-5].
Since the first preliminary study of spinal ultrasonography (US) by Scheible et al. [6], spinal US has been widely used as a screening test in neonates needing evaluation of the spinal cord and/or the FT. The primary US criterion for an abnormal FT is a thickness >2 mm, which would lead to suspicion of fibrolipoma involving the FT [6-13]. The US findings of the normal FT have received little attention in the literature; it has been described as a cordlike, echogenic structure surrounded by the echogenic nerve roots of the cauda equina [12]. With advances in US technology, the normal FT can be described more precisely than as an echogenic cord. In this study, we aimed to establish the US features of the normal FT in infants younger than 6 months of age using high-resolution US transducers.
Materials and Methods
This study was approved by the Institutional Review Board of our hospital. The requirement for informed consent was waived, since this was a retrospective study that was limited to previously obtained imaging data.
We reviewed all spinal US exams performed on infants younger than 6 months of age at a single tertiary hospital between January 2016 and December 2016. Spinal US was performed with a 5-12 MHz linear transducer (Logiq E9, GE Healthcare, Milwaukee, WI, USA) in the prone position, or in the left decubitus position, by two radiologists: one with pediatric fellowship training and 16 years of experience, and the other with pediatric fellowship training and 5 years of experience. These two radiologists examined the spinal cord and FT from the coccyx towards the cranium, as far as the mid-thoracic level. In the patients who cooperated with the examination, video clips were obtained in both the transverse and longitudinal directions. We measured the thickness of the FT near the lumbosacral junction in both the transverse and longitudinal directions.
The images were reviewed by two radiologists in consensus: one with pediatric fellowship training and 16 years of experience, and the other with one year of experience. For this study, we enrolled spinal US images with a normal FT and with video clips, which were necessary for an objective retrospective review.
The two readers analyzed the US features of the normal FT focused on 3 points. First, the readers evaluated the visibility of the internal structure, especially the central canal, of the normal FT on each US scan. Second, the readers compared the marginal echogenicity of the FT with that of the nerve roots of the cauda equina and classified the FT as hyperechoic, isoechoic, or hypoechoic. Third, the readers compared the ability of transverse and longitudinal US images to distinguish the presence of a normal FT from the surrounding nerve bundles of the cauda equina, and determined which orientation was superior.
In a review of the patientsŌĆÖ medical records, age, gender, and the reason for the US examination were recorded. One-way ANOVA and the t test were used to characterize the relationship between each US feature and the thickness of the FT. The Kruskal-Wallis test was used to determine how each US feature was associated with the patientsŌĆÖ age. The chi-square test was used to compare each US feature according to the patientsŌĆÖ gender. All statistical analyses were performed using IBM SPSS software ver. 20 (IBM Corp., Armonk, NY, USA). P-values of <0.05 were considered to indicate statistical significance.
Results
A total of 114 spinal US scans were performed on infants younger than 6 months of age between January and December 2016. Six patients had a filar cyst, and five showed a ventriculus terminalis at the conus medullaris. Two patients showed low-lying conus medullaris that was located below the L3 vertebral body. There was one fibrolipoma of the FT that was confirmed with magnetic resonance imaging. One patient showed decreased pulsation of the spinal cord. Even though the filar cyst and the ventriculus terminalis were not abnormal findings, but congenital variations, we did not enroll them in this study. Among the remaining 99 patients, a total of 30 spinal US scans (14 males, 16 females; mean age, 12 days; range, 1 to 43 days) included video clips in the transverse and longitudinal directions, and these subjects were ultimately included in the study. These 30 spinal US scans were requested due to concerns over skin dimples located in the midline of the sacrococcygeal area. All dimples were shallow and revealed not to be connected with the spinal canal, which is consistent with a simple sacrococcygeal dimple.
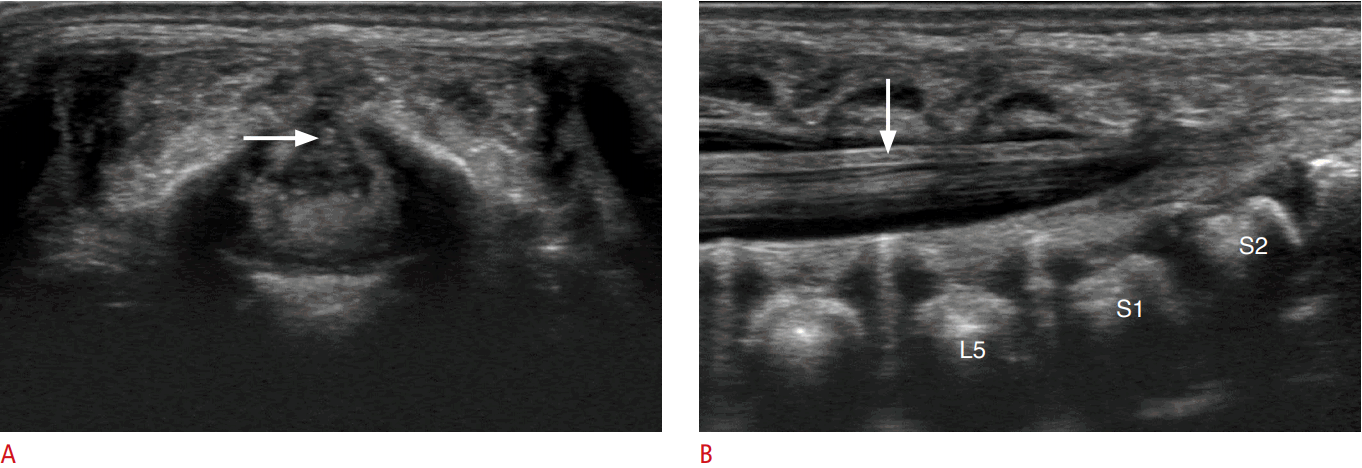
First, the readers evaluated the visibility of the internal structure of the normal FT. In 18 cases (60%), we could define an echogenic line or a tubular structure with echogenic margins in the center of the FT (Fig. 1). This linear or tubular structure continued from the central echo complex of the spinal cord, which indicated the central canal of the FT. In the remaining 12 cases (40%), the FT was a hypoechoic cordlike structure without any visible internal structure. When we performed a statistical analysis of the visibility of the central canal according to the patientsŌĆÖ age and gender, no factors showed statistical significance. The mean thickness of the FT on the transverse scan was 0.09┬▒0.02 mm in patients with a visible internal structure and 0.07┬▒0.02 mm in those without a visible internal structure; this difference did not reach statistical significance (P=0.267). The mean thickness of the FT on the longitudinal scan was 0.09┬▒0.03 mm in patients with a visible internal structure and 0.08┬▒0.02 mm in those without a visible internal structure; this difference likewise did not reach statistical significance (P=0.105).
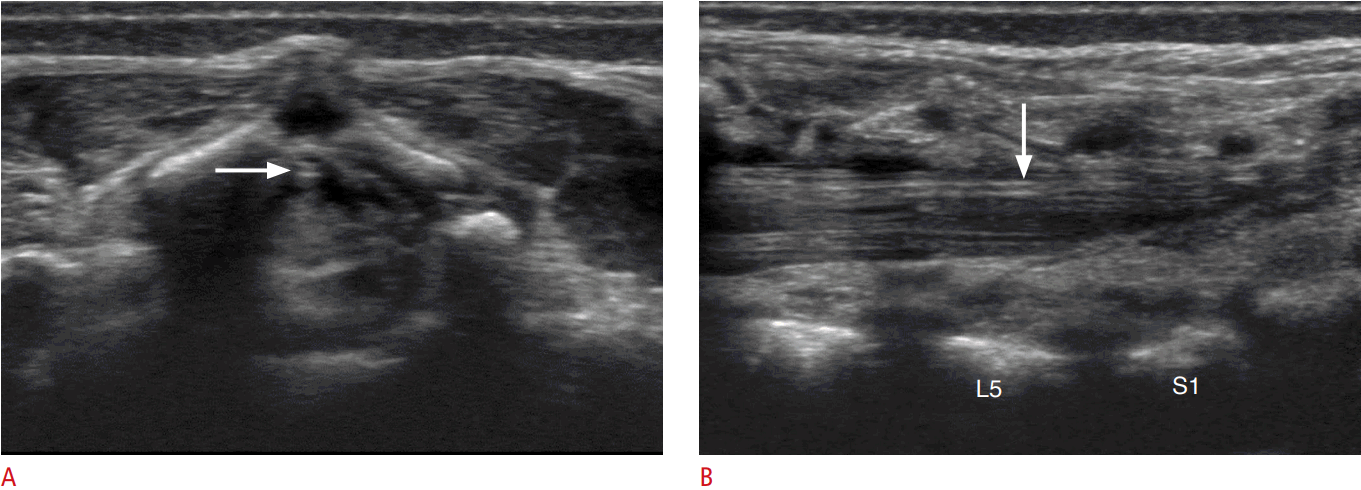
Second, the readers compared the marginal echogenicity of FT with that of the cauda equina. Eight of the FTs (27%) were hyperechoic in comparison to the nerve roots of the cauda equina (Figs. 1, 2). The remaining 22 FTs (73%) were isoechoic, and no FT was hypoechoic. When we performed a statistical analysis of the echogenicity of FT according to the patientsŌĆÖ age and gender, no factors showed statistical significance. The mean thickness of the FT on the transverse scan was 0.09┬▒0.02 mm in patients with a hyperechoic FT and 0.08┬▒0.02 mm in those with an isoechoic FT, and this difference did not reach statistical significance (P=0.895). The mean thickness of the FT on the longitudinal scans was 0.10┬▒0.03 mm in patients with a hyperechoic FT and 0.08┬▒0.02 mm in those with an isoechoic FT, which likewise did not show statistical significance (P=0.266).
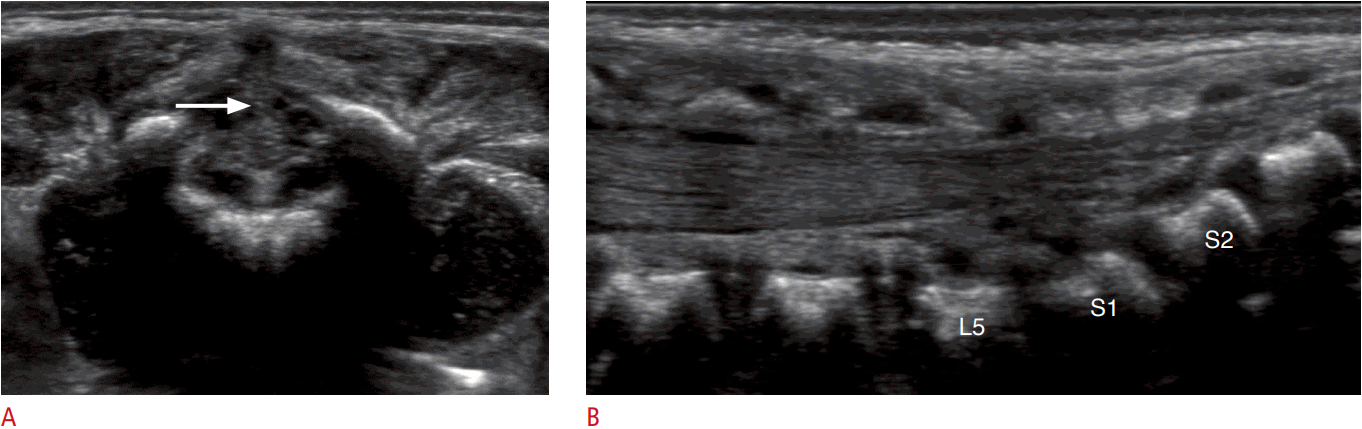
Third, the readers compared transverse US with longitudinal US in terms of the ability to distinguish the normal FT from the surrounding nerve bundles. The transverse scan was superior for differentiating the normal FT in 18 cases (60%) (Fig. 3). The longitudinal scan was superior in two cases (7%), and both directions were rated the same in 10 cases (33%).
Discussion
The caudal end of the spinal cord is the conus medullaris, and it continues into the FT, which extends caudally, as a thin and fibrous filament, to insert into the posterior body of C1. The viscoelasticity of the FT allows the conus medullaris to move slightly during flexion and extension of the spine [14]. For investigations of the spinal cord, FT, cauda equina, and meningeal coverings in the spinal canal, US is regarded as the first-line diagnostic modality in neonates and infants, since at these ages, the posterior vertebral arches are not completely ossified [4,7-12,15].
In the literature, the US features of the spinal cord have been described as involving a hypoechoic tubular structure with a central echo complex, while the FT has been described as a hyperechoic cord [7,10,12]. Since studies have reported that the FT is not just a simple fibrovascular band, but is also composed of glial cells, connective tissue, and remnants of an ependymal-lined central canal [5,16-18], we tried to determine the ultrastructural US features of the normal FT with the help of current advanced US technology, especially with the increased resolution of US transducers. In our results, the normal FT presented as a hypoechoic cordlike structure on US, and the central canal continuing from the central echo complex of the spinal cord was discernible in 60% of normal FTs. In the literature, the central canal of the FT has been reported as only being revealed by microscopic evaluations, especially in the FT internum [3,5,19,20].
The pia mater covering the spinal cord continues down to the FT, as was proven via hematoxylin and eosin staining by Fontes et al. [3]. The pia mater extends along each spinal nerve root and becomes continuous with the connective tissue surrounding each spinal nerve [21]. We presumed that the marginal echogenicity of the FT and the cauda equina in our results were due to the pia mater; however, we still could not determine why 27% of FTs were hyperechoic in comparison to the cauda equina. Nevertheless, the fact that the FT has a more echogenic margin than the cauda equina was helpful for distinguishing the FT from the nerve bundles in cases where it was difficult to identify the normal FT among the congregated or floating nerve bundles of the cauda equina.
We also found that it helped to use transverse US before longitudinal US for distinguishing the FT from the nerve roots, since the transverse scan was superior for differentiating the normal FT in 60% of cases. We speculate as to the reasons for this result as follows: first, the FT is located in the centermost area of this region, while the nerve roots of the cauda equina spread out. Second, longitudinal scans are not obtained at the exact midline, since it is necessary to perform longitudinal scans in a slightly off-midline plane due to the spinous process.
This study has some limitations. First, the sample size was small; therefore, the statistical analysis of associations between the US features and the patientsŌĆÖ information did not show statistical significance. More subjects will be enrolled in a subsequent study. Second, we enrolled US exams that were interpreted as normal. This definition was based on the classic radiological criterion, in which the normal FT is <2 mm thick; however, this may not be the gold standard. According to a study by Selcuki et al. [4], a radiologically normal FT may not always confirm a histopathologically normal FT, and Shin et al. [18], in their retrospective study comparing US with magnetic resonance imaging, reported that the optimal US cutoff value for lipomas in the FT was 1.1 mm. A further prospective study is needed to establish updated radiological features of the normal FT.
In conclusion, the central canal of the FT was visible on US in over half of the normal FTs. Even though the majority of FTs were isoechoic with the nerve roots of cauda equina, 27% were hyperechoic in comparison to the cauda equina, which was helpful to distinguish the FT from the nerve bundles. In addition, transverse US was superior to longitudinal US in distinguishing the normal FT from the nerve roots.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




