AbstractPurposeWe aimed to document the time of onset of ultrasonographic and histologic changes in the testes of a rat model following testicular torsion.
MethodsTwenty-five Sprague-Dawley rats were divided into four groups. All animals underwent preoperative Doppler ultrasonography. Groups 1, 2, and 3 underwent unilateral surgical torsion of the testis lasting for 72, 24, and 6 hours, respectively. Group 4 underwent a sham operation. The animals were followed with Doppler ultrasonography at 6, 24, 48, and 72 hours postoperatively. Histologic examinations were performed at the designated final time point for each group.
ResultsAfter torsion, enlargement of the epididymal head and thickening of the spermatic cord over time were noted. Based on the ultrasonographic dimensions,
the ratio of the epididymal volume increased with time following torsion (p=0.002). The torsed testes had an average weight gain of 0.27 g at 6 hours compared to the control testes, but an average weight loss of 0.22 g at 72 hours (P=0.006). Changes in testicular echotexture were noted as soon as 6 hours after torsion, but there was no consistent pattern of echotexture change thereafter. Histologically, viable tubules were seen 6 hours after torsion, while extensive hemorrhagic necrosis was found at 72 hours.
ConclusionIn evaluating testicular torsion, the enlargement ratio of the epididymis and thickening of the spermatic cord on Doppler ultrasonography may be useful for determining the urgency of immediate surgery. Changes in testicular echotexture may not be a reliable indicator of the time of onset.
IntroductionTorsion of the testis is the most common urologic emergency in children and young adults, and patients younger than 18 years are especially prone to testicular loss because of a median delay in medical evaluation of 20 hours after the onset of scrotal pain [1]. In these patients, the reported time of onset of scrotal pain has been used as a factor to determine the need for immediate surgery and the prognosis for testicular salvage. Irreversible ischemic injury to the testicle can be inflicted as early as 4 hours after the onset of testicular torsion [2]. Therefore, urgent surgical exploration is recommended in all cases of testicular torsion within 24 hours of symptom onset [3,4]. However, the time of onset of testicular torsion is difficult to determine by history alone, as it has been found to be inaccurate in up to 50% of patients [5].
Ultrasonography, including conventional gray-scale and color Doppler imaging, has become an important diagnostic tool in the evaluation of acute conditions of the scrotum because of its widespread availability, noninvasiveness, and excellent accuracy (>95%) in the diagnosis of testicular torsion and testicular ischemia [6,7]. Some sonographic features have been suggested to be a reliable sign for diagnosing torsion. The spermatic cord "whirlpool sign," which refers to a spermatic cord with an abrupt spiral twist at the external inguinal ring or in the scrotal sac, is considered to be highly diagnostic for torsion [8,9]. Testicular or epididymal enlargement, heterogeneous parenchymal echotexture, redundant spermatic cord, and horizontal lie of the affected testis are also associated with torsion [8-11].
In previous studies, researchers have described the use of rat models to identify sonographic and pathologic features of testicular torsion [12-14]. A hypoechoic, inhomogeneous testis and reduced intratesticular blood flow have been suggested as predictive factors of nonviability of the testis after torsion [12]. However, sonographic and histologic features useful for estimating the time of torsion onset have not been fully elucidated. The objective of this study was to evaluate the usefulness of specific findings on Doppler ultrasonography and histologic characteristics for estimating the time of onset of testicular torsion and predicting testicular viability in an animal model of unilateral testicular torsion.
Materials and MethodsTwenty-five adult male Sprague-Dawley rats (250-390 g) were obtained from university vivarium sources. The animals were given free access to pellet food and water and were housed in a temperature- and light-controlled room. Approval for the experimental protocol was obtained from the Institutional Animal Care and Use Committee of University of Southern California.
Surgical TorsionAll surgical procedures were performed under anesthesia induced by the inhalation of isoflurane (1%-2%). Testicular torsion was performed on the left testis through a lateral scrotal incision. Testicular torsion was created by delivering the left testis through the incision, rotating the left testis 720┬░ counterclockwise, and fixing the torsed testis to the lateral scrotal wall with a 4-0 polyglactin suture. The right hemiscrotum was left intact so that the right testis could serve as a control. In the sham operations, the left testis was delivered through the incision without torsion. After each surgical intervention, the incisions were temporarily closed using 4-0 polyglactin sutures.
The animals were randomly separated into four groups. All animals underwent preoperative scrotal ultrasonography. Group 1 (72-hour torsion) underwent surgical torsion, and then underwent scrotal ultrasonography at 0, 6, 24, 48, and 72 hours postoperatively. Group 2 (24-hour torsion) underwent surgical torsion, and then underwent scrotal ultrasonography at 0 hour, 6 hours, and 24 hours postoperatively. Group 3 (6-hour torsion) underwent surgical torsion, and then underwent scrotal ultrasonography at 0 hour and 6 hours postoperatively. Group 4 (the control group) underwent the sham operation, and then underwent scrotal ultrasonography at 0, 24, 48, and 72 hours postoperatively. At the final time point for each group, en bloc harvests of both testicles and epididymides were performed.
Imaging TechniqueImaging was performed anesthesia induced by the inhalation of isoflurane (1%-2%) with an Acuson Sequoia 512 ultrasound system (Acuson, Mountain View, CA, USA). Conventional gray-scale scrotal sonography with a 7.0-14.0 MHz linear phased-array transducer (model 15L8, Acuson), followed immediately by color Doppler imaging, was performed by an ultrasound technician, with oversight by a pediatric radiologist. Imaging was routinely performed in the longitudinal and transverse planes, and the dimensions of each testis and epididymal head were recorded (Fig. 1). Testis volume was calculated as (height├Ślength├Świdth├ŚŽĆ/6) [15].
Histologic ExaminationsThe testicular specimens were weighed and embedded in paraffin blocks. Sections were obtained at 5 ╬╝m and stained with hematoxylin and eosin. Histologic changes in viable tubules and the area of necrosis in the testes were counted by a pathologist in a blinded fashion under an optical microscope.
Statistical AnalysisThe paired t-test was used to compare testicular weight, epididymal and testicular volume ratio between the torsed and contralateral controls. The F test of group-time interaction in analysis of variance (ANOVA) with rats as a random effect and time as a repeated measure (using a split-plot ANOVA to calculate the sum square) was used to compare the epididymal and testicular volumes. Volume was calculated as the product of length├Świdth├Śheight. P-values of <0.05 were considered to indicate statistical significance.
ResultsAll testes were preoperatively confirmed to have evidence of intratesticular arterial blood flow on color Doppler imaging. The absence of intratesticular arterial blood flow on color Doppler imaging was noted in all torsed testes immediately following the onset of surgical torsion. In addition, color Doppler imaging confirmed the presence of intratesticular arterial blood flow in all right control testes, as well as in the testes of the sham operation group.
With increasing time following torsion, marked heterogeneous, mass-like enlargement of the epididymal head and thickening of the spermatic cord were noted, but not in Group 4. Based on the ultrasonographic dimensions, the enlargement ratio of the epididymal volume (ratio of the torsed epididymal volume to the control epididymal volume) was 1.94 (95% confidence interval [CI], 1.59 to 2.36) after 6 hours of torsion (Fig. 2). After 72 hours of torsion, this ratio increased to 3.11 (95% CI, 2.18 to 4.42). The overall P-value was 0.002.
The torsed testes had an average weight gain of 0.27 g (95% CI, 0.06 to 0.48) after 6 hours of torsion, as compared to the control testes, but showed an average weight loss of 0.22 g (95% CI, 0.07 to 0.36) after 72 hours of torsion (Fig. 3). The overall P-value was 0.006.
Sonographically, prior to surgery, all testes were noted to have a uniform, medium-level, homogeneous echogenicity, which is a similar pattern of ultrasound characteristics to those found in humans. In the torsed testes, sonographic changes including hyperechogenicity and a heterogeneous echotexture were noted as soon as 6 hours after complete torsion. However, after 72 hours of torsion, most of the torsed testes appeared heterogeneous, with either marked hyperechogenicity or hypoechogenicity. No consistent patterns of echotexture change over time were identified.
Based on the dimensions measured on ultrasonography, no statistically significant differences in testicular volume between the torsed testes and the control testes were measured over the 72-hour period of torsion (Fig. 4). The overall P-value was 0.074.
Histologically, viable tubules with intercellular edema and diffuse interstitial congestion and hemorrhage were consistently seen after 6 hours of torsion, while extensive hemorrhagic necrosis was consistently found after 72 hours of torsion (Fig. 5).
DiscussionPrompt evaluation and treatment are essential for salvaging testicular function after testicular torsion and avoiding testicular loss. Turner and Brown [16] have previously shown in animal models that longer periods of testicular torsion are associated with increased damage to the testis. In the clinical setting, it is reported that the testicular salvage rate is almost 100% if surgery is performed within 6 hours of presentation and less than 20% when surgery is deferred to after 12 hours [17]. Bartsch et al. [2] showed that 50% of patients with testicular torsion-despite detorsion within 4 hours-may suffer from a future decrease in testicular exocrine function. Abnormal follicle-stimulating hormone and inhibin B levels have also been demonstrated in 35% of patients with testicular torsion either at early or late follow-up examinations [18]. In addition to the possibility of testicular loss on the affected side, previous studies have shown that the contralateral testis may also be affected [13,19-21]. This can result in future sequelae regarding the hormonal levels, sexual function, and fertility status of affected patients.
The time between the onset of symptoms and detorsion and the degree of cord twisting are known to be the two most important determinants of early salvage of the testis [3]. However, upon presentation to the emergency room, it is difficult for the clinician to determine the time of onset of testicular torsion since the clinical history is often unreliable [5]. Immediate surgery may not be feasible for every presentation of acute scrotal pain for logistical reasons, and therefore accurate assessment of time post-torsion can help the surgeon gauge the urgency of surgery.
Gray-scale or color Doppler ultrasonography is an effective imaging tool to evaluate acute conditions involving the scrotum, such as testicular torsion. Kalfa et al. [22] reported that the presence of a twisted spermatic cord was a highly sensitive and specific sign of testicular torsion. Prando [9] showed that a diffusely hypoechoic testis without detectable arterial and venous testicular flow was a diagnostic feature of acute testicular ischemia. Color Doppler ultrasonography has been reported to be highly accurate for diagnosing torsion, with a sensitivity of 63.6%-100% and a specificity of 97%-100% [6,22,23]. However, little is known regarding the association of ultrasonographic features with the duration of torsion.
A previously underutilized feature of scrotal ultrasonography, the epididymal enlargement ratio, may offer additional important clinical information that is easily obtained using currently available ultrasonographic equipment. In this study, an epididymal enlargement ratio of approximately 3-meaning that the epididymis on the affected side was 3 times larger than the epididymis on the control side-was associated with longer periods of testicular torsion and correlated with non-viable testes on histologic evaluations. A lower enlargement ratio was associated with a shorter period of testicular torsion and with viable testes. This finding is consistent with a previous report showing that the vast majority of patients with testicular torsion showed enlarged epididymal volume and hyperechogenicity on ultrasonography [9,11]. Galina et al. [24] also focused on the abnormal configuration, size, and displaced position of the epididymis in patients with torsion and suggested that careful ultrasonographic evaluation of the epididymis may be useful as an adjunctive tool to diagnose testicular torsion.
We demonstrated testicular hyperechogenicity and heterogeneity as early as 6 hours after complete torsion, but found no consistent patterns of echotexture change beyond 6 hours, whereas a previous study found that preoperative heterogeneous parenchymal echotexture indicated late torsion and testicular nonviability [10]. In the early days of using ultrasonography for the diagnosis of acute conditions involving the scrotum, focal hyperechogenicity was mentioned as evidence of chronic torsion [25]. Currently, it is believed that torsed testes may be hypoechoic from edema or hyperechoic from hemorrhage [26]. Liang et al. [27] reported in their retrospective review that although a heterogeneous testis on ultrasonography was common in cases of torsion (present in 80% of cases), heterogeneity was not a good predictor of necrosis. Our result also showed various echogenicity patterns over time after torsion, which concurs with previous reports. Of note, the histologic evaluation of testes in our animal model after 6 hours of testicular torsion revealed viable tubules. Therefore, we also suggest that clinicians should not rely on echogenicity alone to diagnose testicular torsion.
This study has several limitations. Firstly, in this study, the histologic assessment of the torsed testis was based on the pathologistŌĆÖs reading, not on an objective evaluation using a grading system. Rather than using a grading system to evaluate tubule and germinal cells, our result focused more on macroscopic and geometric changes, such as weight and volume of the torsed testis over time. Therefore, our results are limited in terms of their implications for changes in germ cells and spermatogenesis after torsion. Secondly, although our result concurs with previous studies describing epididymal ultrasonographic changes in patients with testicular torsion, [9,11], such findings will be more meaningful when they are correlated with histologic findings, and ultimately, with the true long-term function of the affected testes. Thus, we acknowledge that further studies are necessary to determine the clinical utility of the epididymal enlargement ratio based on ultrasonographic findings.
Testicular atrophy is the main concern after testicular torsion. In a meta-analysis, the mean late testicular loss rate was nearly 50% [28]. However, only a few studies have reported the onset of testicular atrophy after torsion. In an experimental rat torsion model, even 3 hours of torsion resulted in some degree of atrophy of the testis and epididymis, causing reduced sperm motility and concentration [29]. Saba et al. [30] showed that testis weight was not affected by 1 or 2 hours of torsion or at 24 hours after detorsion. However, they found a 45% decrease in testis weight after torsion and at 1 week after detorsion. In our study, we demonstrated atrophy of the testis through measurements of testicular weight, which decreased as early as 72 hours compared to testicular weight in the control group. Because the volume and histological features of the contralateral testis of a torsed testis are also affected [13,31], we recognize that a comparison of volume between a torsed testis and the contralateral testis is not optimal for demonstrating testicular atrophy in the acute phase of testicular torsion.
In the evaluation of acute conditions of the scrotum, a previously underutilized ultrasonographic feature, the enlargement ratio of the epididymis, may help in estimating the time of onset of testicular torsion. In a clinical setting, this information may be useful for determining the urgency of immediate surgery and the prognosis for testicular salvage. Changes in testicular echotexture do not appear to be a reliable indicator of the time of onset of spermatic cord torsion.
NotesAuthor Contributions Conceptualization: Hung AJ, Koh CJ. Data acquisition: Song SH, Af┼¤arlar ├ćE, Xie HW, Hung AJ, Koh CJ. Data analysis or interpretation: Song SH, Af┼¤arlar ├ćE, Xie HW, Hung AJ, Koh CJ. Drafting of the manuscript: Song SH, Af┼¤arlar ├ćE, Xie HW, Hung AJ, Koh CJ. Critical revision of the manuscript: Song SH, Koh CJ. Approval of the final version of the manuscript: all authors. AcknowledgementsWe thank Dr. Susan L. Groshen and Denise Wei for their assistance with the statistical analysis, Dr. Marguerite T. Parisi and Alice Wong for their assistance with ultrasonography, and Dr. Ignacio Gonzalez-Gomez for his assistance with pathology review.
References1. Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol 1989;142:746ŌĆō748.
2. Bartsch G, Frank S, Marberger H, Mikuz G. Testicular torsion: late results with special regard to fertility and endocrine function. J Urol 1980;124:375ŌĆō378.
3. Visser AJ, Heyns CF. Testicular function after torsion of the spermatic cord. BJU Int 2003;92:200ŌĆō203.
4. Tryfonas G, Violaki A, Tsikopoulos G, Avtzoglou P, Zioutis J, Limas C, et al. Late postoperative results in males treated for testicular torsion during childhood. J Pediatr Surg 1994;29:553ŌĆō556.
5. Herbener TE. Ultrasound in the assessment of the acute scrotum. J Clin Ultrasound 1996;24:405ŌĆō421.
6. Gunther P, Schenk JP, Wunsch R, Holland-Cunz S, Kessler U, Troger J, et al. Acute testicular torsion in children: the role of sonography in the diagnostic workup. Eur Radiol 2006;16:2527ŌĆō2532.
7. Yagil Y, Naroditsky I, Milhem J, Leiba R, Leiderman M, Badaan S, et al. Role of Doppler ultrasonography in the triage of acute scrotum in the emergency department. J Ultrasound Med 2010;29:11ŌĆō21.
8. Munden MM, Williams JL, Zhang W, Crowe JE, Munden RF, Cisek LJ. Intermittent testicular torsion in the pediatric patient: sonographic indicators of a difficult diagnosis. AJR Am J Roentgenol 2013;201:912ŌĆō918.
9. Prando D. Torsion of the spermatic cord: the main gray-scale and doppler sonographic signs. Abdom Imaging 2009;34:648ŌĆō661.
10. Kaye JD, Shapiro EY, Levitt SB, Friedman SC, Gitlin J, Freyle J, et al. Parenchymal echo texture predicts testicular salvage after torsion: potential impact on the need for emergent exploration. J Urol 2008;180:1733ŌĆō1736.
11. Nussbaum Blask AR, Rushton HG. Sonographic appearance of the epididymis in pediatric testicular torsion. AJR Am J Roentgenol 2006;187:1627ŌĆō1635.
12. Kwon JW, Kim WS, Cheon JE, Kim CJ, Kim IO, Yeon KM. Evaluation of testicular viability by power Doppler ultrasonography in experimentally induced acute testicular torsion. Invest Radiol 2005;40:682ŌĆō687.
13. Oh SJ, Kwak C, Baek M, Kim CS, Kim KS, Choi H. Histologic and molecular changes in the ipsilateral and contralateral epididymides of the rat in response to unilateral testicular torsion followed by detorsion. Fertil Steril 2004;81 Suppl 1:882ŌĆō887.
14. Herek D, Herek O, Akbulut M, Ufuk F. Role of strain elastography in the evaluation of testicular torsion: an experimental study. J Ultrasound Med 2016;35:2149ŌĆō2158.
15. al Salim A, Murchison PJ, Rana A, Elton RA, Hargreave TB. Evaluation of testicular volume by three orchidometers compared with ultrasonographic measurements. Br J Urol 1995;76:632ŌĆō635.
16. Turner TT, Brown KJ. Spermatic cord torsion: loss of spermatogenesis despite return of blood flow. Biol Reprod 1993;49:401ŌĆō407.
17. Sung EK, Setty BN, Castro-Aragon I. Sonography of the pediatric scrotum: emphasis on the Ts: torsion, trauma, and tumors. AJR Am J Roentgenol 2012;198:996ŌĆō1003.
18. Taskinen S, Taskinen M, Rintala R. Testicular torsion: orchiectomy or orchiopexy? J Pediatr Urol 2008;4:210ŌĆō213.
19. Jeong SJ, Choi WS, Chung JS, Baek M, Hong SK, Choi H. Preventive effects of cyclosporine a combined with prednisolone and melatonin on contralateral testicular damage after ipsilateral torsion-detorsion in pubertal and adult rats. J Urol 2010;184:790796.
20. Schanaider A, Aiex CA, Errico G. Immunological effects of acute testicular torsion on the contralateral testis in rats. Eur J Pediatr Surg 2011;21:370ŌĆō374.
21. Wei SM, Yan ZZ, Zhou J. Involvement of reactive oxygen species and TATA box-binding protein-related factor 2 in testicular torsion/ detorsion-induced injury. Urology 2013;81:466.
22. Kalfa N, Veyrac C, Lopez M, Lopez C, Maurel A, Kaselas C, et al. Multicenter assessment of ultrasound of the spermatic cord in children with acute scrotum. J Urol 2007;177:297ŌĆō301.
23. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics 2000;105(3 Pt 1):604ŌĆō607.
24. Galina P, Dermentzoglou V, Baltogiannis N, Zarifi M. Sonographic appearances of the epididymis in boys with acute testicular torsion but preserved testicular blood flow on color Doppler. Pediatr Radiol 2015;45:1661ŌĆō1671.
25. Chinn DH, Miller EI. Generalized testicular hyperechogenicity in acute testicular torsion. J Ultrasound Med 1985;4:495ŌĆō496.
27. Liang T, Metcalfe P, Sevcik W, Noga M. Retrospective review of diagnosis and treatment in children presenting to the pediatric department with acute scrotum. AJR Am J Roentgenol 2013;200:W444ŌĆōW449.
28. MacDonald C, Kronfli R, Carachi R, O'Toole S. A systematic review and meta-analysis revealing realistic outcomes following paediatric torsion of testes. J Pediatr Urol 2018;14:503ŌĆō509.
29. Merimsky E, Rock M, Katz S. Assessment of fertility after testicular torsion: an experimental study. Urol Res 1982;10:51ŌĆō54.
Representative longitudinal ultrasonography in the surgical (A, B) and control (C, D) groups.Gray-scale with Doppler ultrasonography shows measurements of testicular (A) and epididymal dimensions (B) in the surgical (A, B) groups. The cephalo-caudal length (distance number 1) and anteroposterior length (distance number 2) were measured on a longitudinal view of the rat's testis and epididymis to calculate the epididymis-to-testis volume ratio.
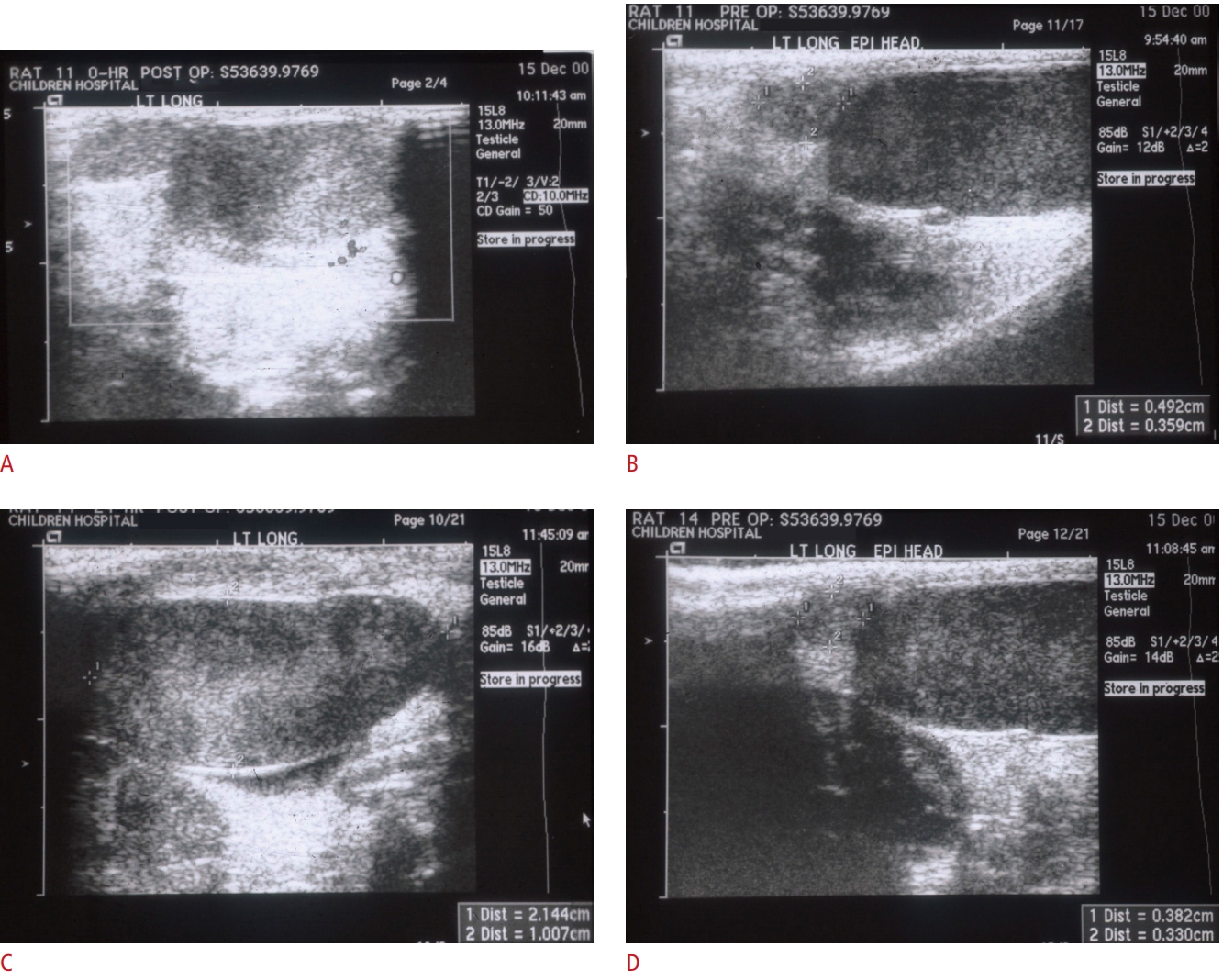 Fig.┬Ā1.Ratio of epididymal volumes by ultrasonography [left (torsion)/right (control)].Based on the ultrasonographic dimensions, the enlargement ratio of the epididymal volume increased with time following torsion.
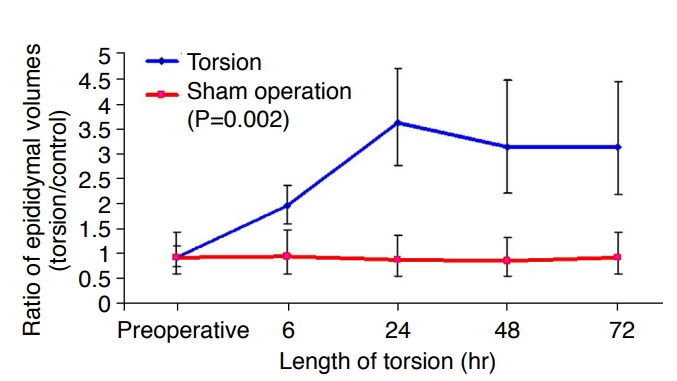 Fig.┬Ā2.Mean difference in testis weight [left (torsion)-right (control)].The torsed testes had an average weight gain after 6 hours of torsion, but an average weight loss after 72 hours of torsion.
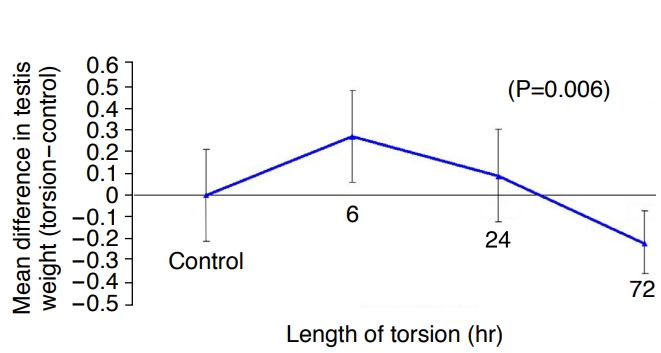 Fig.┬Ā3.Ratio of testicular volumes by ultrasonography [left (torsion)/right (control)].Based on the dimensions measured on ultrasonography, no statistically significant differences in testicular volume between the torsed testes and the control testes were measured over the 72-hour period of torsion.
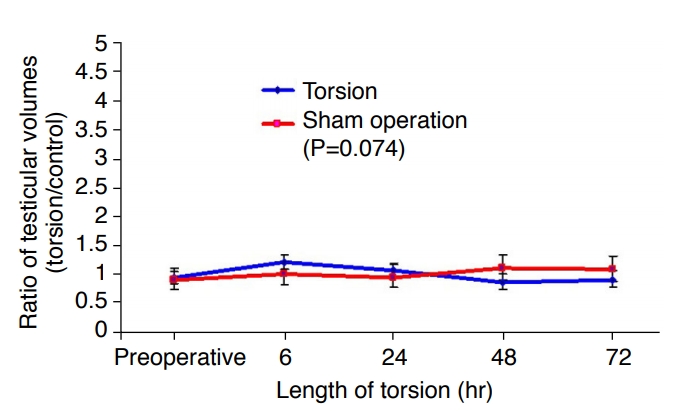 Fig.┬Ā4.Histologic slides.A. Normal tubule formation in the control group was observed. B. Viable tubules with intercellular edema and diffuse interstitial congestion and hemorrhage were observed after 6 hours of torsion. C. Testicular viability significantly diminished after 24 hours of torsion. D. Extensive hemorrhagic necrosis was found after 72 hours of torsion (A-D, H&E, ├Ś10).
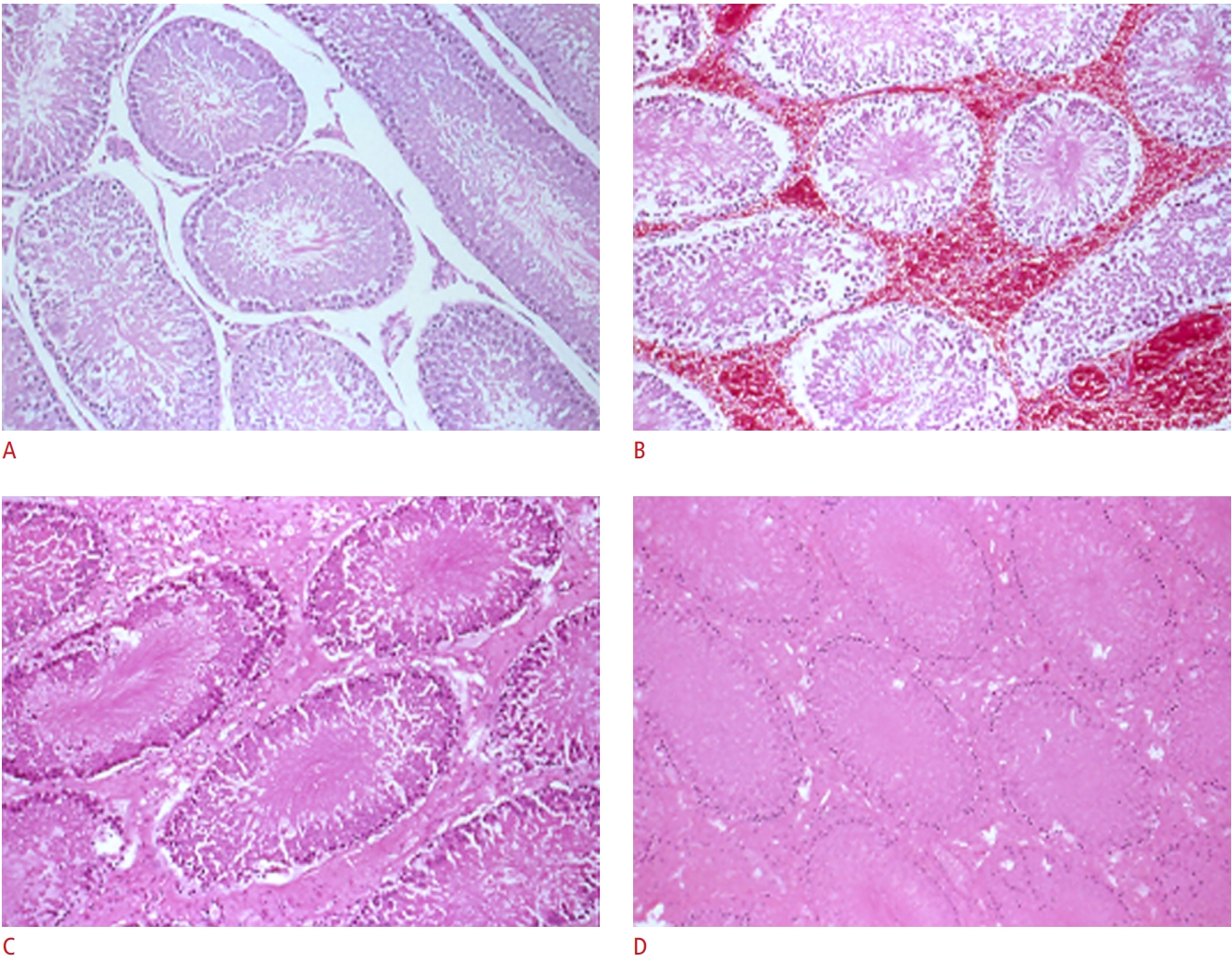 Fig.┬Ā5. |