AbstractPurposeThis study aimed to evaluate local tumor progression-free survival (LTPFS) and overall survival (OS) after percutaneous radiofrequency ablation (RFA) for solitary colorectal liver metastases (CLM) <3 cm and to identify the risk factors associated with poor LTPFS and OS after percutaneous RFA.
MethodsThis study screened 219 patients who underwent percutaneous RFA for CLM between January 2013 and November 2020. Of these, 92 patients with a single CLM <3 cm were included. LTPFS and OS were calculated using the Kaplan-Meier method, and the differences between curves were compared using the log-rank test. Risk factors for LTPFS and OS were assessed using Cox proportional-hazard regression models.
ResultsTechnical efficacy was achieved in the first (n=91) or second (n=1) RFA sessions. During the follow-up (median, 20.0 months), cumulative LTPFS rates at 1, 3, and 5 years were 92.4%, 83.4%, and 76.5%, respectively. During the follow-up (median, 27.8 months), the corresponding OS rates were 97.5%, 81.3%, and 74.8%, respectively. In multivariable Cox regression analyses, the group with both tumor-puncturing RFA and a T4 stage primary tumor (hazard ratio, 3.3; 95% confidence interval, 1.1 to 10.2; P=0.037) had poor LTPFS. In the univariable analysis, no factors were significantly associated with poor OS.
IntroductionRadiofrequency ablation (RFA) has been used to manage metastatic colorectal cancer (CRC) in the liver of patients ineligible for hepatic resection. However, one of the drawbacks of RFA is frequent local tumor progression (LTP) compared to hepatic resection. Tumor size, ablative margin, and perivascular or subcapsular tumor location have been associated with LTP after RFA [1-3].
Meanwhile, tumors up to 3 cm in diameter are generally considered good candidates for RFA of colorectal liver metastases (CLM) [4,5]. As RFA has evolved in the last decade, RFA with multiple or perfusion electrodes is currently being used to create a sufficient ablative margin. Recently, no-touch RFA, wherein multiple radiofrequency electrodes are placed outside the tumor, has become popular. As no-touch RFA can create a larger ablation zone than conventional tumor-puncturing RFA [6], it is easy to create ablative margins larger than 1.0 cm all around the index tumor, which are ideal for CLM [3,7,8]. This could ensure excellent local tumor control.
Furthermore, it is expected that even perivascular tumors, which are prone to the so-called "heat-sink effect," may be overcome by these advanced RFA techniques as multiple electrodes can be placed outside the tumor near the peritumoral vessels. In addition, more accurate guidance methods, such as fusion imaging of real-time ultrasonography (US) and pre-acquired computed tomography (CT), magnetic resonance imaging (MRI), or contrast-enhanced US, are now available [9-11]. For these reasons, therapeutic outcomes after RFA using advanced techniques need to be re-assessed using the latest clinical data. However, to the authorsŌĆÖ knowledge, no studies of RFA for CLM have reflected these recent changes.
Therefore, this study aimed to evaluate LTP-free survival (LTPFS) and overall survival (OS) after percutaneous RFA for solitary CLM <3 cm at a tertiary cancer center. The risk factors associated with poor LTPFS and OS after percutaneous RFA were also assessed.
Materials and MethodsCompliance with Ethical StandardsThe Institutional Review Board of Samsung Medical Center (2021-11-100) of the authorsŌĆÖ medical center approved this retrospective study, and the requirement for written informed consent from patients was waived.
PatientsIn total, 219 patients who underwent RFA for hepatic metastasis between January 2013 and November 2020 were screened retrospectively. Among them, 127 patients were excluded for the following reasons: (1) multiple metastases (n=119), (2) metastasis from cancers other than CRC (n=5), (3) tumor size >3 cm (n=1), (4) patients who underwent laparoscopic RFA (n=1), and (5) absence of electronic medical records (n=1). Finally, 92 patients who underwent US-guided RFA for a single CLM <3 cm were included. Hepatic metastasis was diagnosed based on percutaneous biopsy (n=2) or typical imaging findings of CT or MRI (n=90) (Fig. 1).
Percutaneous RFAAll RFA procedures were performed percutaneously by five radiologists who had at least 3 years of experience with RFA. The RFA procedures were performed under conscious sedation or monitored anesthesia, which was conducted under the care of anesthesiologists. Fusion imaging (volume navigation; LOGIQ E9, GE Healthcare, Chicago, IL, USA) of real-time US and CT/MRI scans were used to guide and monitor the RFA procedures [12]. Various RFA systems (VIVA RFA System, STARmed, Goyang, Korea; Jet-tip RFA System, RF Medical, Seoul, Korea) were used according to the operatorŌĆÖs preference. The aim was to achieve at least a 0.5- to 1.0-cm ablative margin around the index tumor, except in perivascular and subcapsular tumor locations.
The type and number of radiofrequency electrodes were chosen based on the tumor size and shape. In the earlier period of this study, conventional tumor-puncturing RFA was performed using a single electrode, especially for small tumors <2 cm. When using multiple electrodes (Octopus electrode, STARmed; Twins electrodes, RF Medical), centripetal RFA was performed after placing electrodes at the peripheral portion of the tumor. Gradually, no-touch RFA also started to be used as it can provide a sufficient ablative margin, leading to effective local tumor control [13]. Initially, no-touch RFA was feasible using a monopolar mode of energy delivery. The bipolar mode became available for Twins electrodes in 2018 and Octopus electrodes in 2019, respectively, and after those time points, monopolar, bipolar, or combined monopolar and bipolar modes were used. Overlapping ablation was performed when required to achieve a sufficient ablative margin. RFA procedures were continued until the echogenic zone created by RFA in the US was large enough to cover the entire tumor. The electrode path was cauterized during electrode removal at the end of the RFA procedure.
Assessment of Treatment Response and ComplicationThe treatment response and complications were evaluated using contrast-enhanced CT within 12 hours after the procedure. The treatment was considered a technical success if the RFA zone entirely covered the index tumor on CT [14]. A second RFA session was attempted within 24 hours after the first RFA session when a residual unablated tumor was noted on CT images. Technical efficacy was assessed through 1-month follow-up CT or MRI.
A major complication was defined as an event that led to substantial morbidity and disability that increased the level of care, resulting in hospital admission or a substantially lengthened hospital stay. All other complications were considered minor [14].
Follow-upFollow-up contrast-enhanced CT/MRI was performed 1 month after the initial treatment and every 3 to 6 months thereafter. Chest CT, serum carcinoembryonic antigen, carbohydrate antigen 19-9, and liver function tests were also performed. LTP was defined as the appearance of tumor foci at the edge of the ablation zone [14].
Assessment of Therapeutic OutcomesThe size of the ablation zone was measured on immediate post-RFA CT. The maximum (Dmax) and minimum (Dmin) diameters of the ablation zone were measured from axial CT images of the portal phase and the longest vertical diameter (Dv) from sagittal or coronal images. The ablation volume was calculated by the formula for ellipsoid lesion (ablation volume=ŽĆ(Dmax├ŚDmin├ŚDv)/6) [17].
Therapeutic outcomes, including LTPFS and OS, were assessed. LTPFS was defined as the time interval between the initial RFA and the first radiographic evidence of LTP [5,18]. OS was defined as the time from the initial treatment to death or the last date of a follow-up visit before November 22, 2020.
Statistical AnalysesPatient demographics and clinical characteristics were analyzed using descriptive statistics. They were presented as numbers with percentages for categorical variables and medians with ranges or means with standard deviations for continuous variables. The ratios of the longest diameter of the ablation zone and tumor size and those of the ablation volume and tumor volume were compared between the no-touch RFA and tumor-puncturing RFA groups using the Wilcoxon rank-sum test. Survival curves were generated using the Kaplan-Meier method, and differences between curves were compared using the log-rank test.
Univariable and multivariable analyses were performed to identify risk factors for LTPFS or OS using Cox proportional-hazard regression models. The risk factors for LTPFS were assessed using the variables with patient-, tumor-, and RFA-related factors. The interaction effect between the T stage and the method of electrode placement was assessed through a post-hoc analysis to evaluate the interaction effect of the two variables on LTPFS. Variables with P<0.1 in the univariable analysis and a combination of the two variables related to the interaction effect were included in the multivariable analysis.
The risk factors for OS were also assessed using a Cox regression analysis with the same variables as for LTPFS. The interaction effect between the T stage and the method of electrode placement was also assessed with post-hoc analysis to evaluate the interaction effect of the two variables on OS. Statistical analyses were performed using R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P<0.05.
ResultsBaseline CharacteristicsThe baseline characteristics of the patients are summarized in Table 1. Of the 92 patients included, 68.5% were men. The mean patient age was 60.2 years. The T and N stages of primary CRC were as follows: T1-T3 (70.7%), T4 (27.2%), N0 (31.5%), and N1-N2 (65.2%), respectively. After primary tumor control of CRC, the disease-free interval was Ōēż12 months in 76.1% of patients. The number of radiofrequency electrodes used for RFA was as follows: 1 (32.6%, 30/92), 2 (44.6%, 41/92), and 3 (22.8%, 21/92). The number of overlapping ablations was as follows: 1 (19.6%, 18/92), 2 (21.7%, 20/92), and 3 or more (58.7%, 54/92). In terms of electrode placement, no-touch RFA was performed in 22.8% of patients, and tumor-puncturing RFA was performed in 77.2%.
Treatment Response and ComplicationsTechnical success was achieved in 91 of 92 patients (98.9%). A residual unablated tumor was found in one patient on immediate post-RFA CT images, and a second RFA session was performed, which led to successful local tumor control. Technical efficacy was achieved in all 92 patients (100%) in the 1-month follow-up CT images.
Major complications were found in three patients after RFA. These included biliary sepsis (n=1), subcapsular hematoma (n=1), and large biloma (n=1). All three patients recovered fully after conservative treatment. Minor complications were found in four patients: biloma (n=1), subsegmental infarction (n=2), and diaphragmatic thermal injury (n=1).
Ablation ZoneThe median longest diameter of the ablation zone was 4.6 cm (range, 3.1 to 6.3 cm) in the no-touch RFA group and 3.9 cm (range, 1.9 to 7.3 cm) in the tumor-puncturing RFA group. The median volume of the ablation zone was 24.5 cm3 (range, 9.5 to 58.1 cm3) in the no-touch RFA group and 16.3 cm3 (range, 2.7 to 131.7 cm3) in the tumor-puncturing RFA group (P=0.016). The ratio of the longest diameter and the volume between the ablation zone and those of the tumors between the no-touch RFA and tumor-puncturing RFA groups are summarized in Table 2. The ratio of the longest diameter was significantly larger in the no-touch RFA group than the tumor-puncturing RFA group (P=0.035). The volume ratio had a tendency to be larger in the no-touch RFA group than in the tumor-puncturing RFA group, but it did not reach statistical significance (P=0.114).
Local Tumor Progression-Free SurvivalThe median follow-up period was 20.0 months (range, 1.8 to 90.3 months). During follow-up, LTP was observed in 12 of 92 patients (13.0%). The cumulative LTPFS rates at 1, 3, and 5 years were 92.4%, 83.4%, and 76.5%, respectively.
The univariable Cox proportional hazard regression model revealed that the T stage of the primary tumor was a risk factor for LTPFS (P=0.048). The method of electrode placement (P=0.071) and patient age (P=0.066) showed borderline significance as risk factors for LTPFS (Table 3, Figs. 2, 3). As LTPFS tended to be higher with no-touch RFA than with tumor-puncturing RFA, the possibility of an interaction effect between the method of electrode placement and the T stage of the primary tumor was evaluated, and an interaction effect was found between the two variables. The combined group of tumor-puncturing RFA and a T4 stage tumor was associated with poor LTPFS (hazard ratio, 10.3; 95% confidence interval [CI], 1.2 to 1,345.5; P=0.029), compared to the reference group, no-touch RFA and T1-T3 stage (Table 4, Fig. 4). Multivariable Cox regression analyses showed that the combination of tumor-puncturing RFA and a T4 stage tumor was associated with poor LTPFS (hazard ratio, 3.3; 95% CI, 1.1 to 10.2; P=0.037) (Table 3).
DiscussionThis study analyzed the treatment outcomes of percutaneous RFA of a single CLM <3 cm in size. The results showed an interaction effect of the method of electrode placement and the T stage of primary CRC on LTPFS. The group with both tumor-puncturing RFA and a T4 stage tumor had poorer LTPFS than those with no-touch RFA and T1-T3 stages. The multivariable analysis also showed that the combination of tumor-puncturing RFA and a T4 stage tumor was an independent risk factor for poor LTPFS. However, no interaction effect of the variables mentioned above was found on OS, and there were no significant risk factors associated with poor OS.
A sufficient ablative margin has been reported to be critical for preventing LTP after RFA [1,8,19,20]. When ablative margins were >10 mm, no LTP was found after the RFA of CLM [3,5]. During the study period between January 2013 and November 2020, we became aware of this fact and gradually adopted centripetal RFA or no-touch RFA using multiple electrodes to create a sufficient ablative margin. For this reason, multiple electrodes were used in 80.4% (74/92) of patients in this study, and no-touch RFA was performed in 22.8% (21/92). Of note, LTP was not found in patients who underwent no-touch RFA, which may be attributed to the larger size of the ablation zone with no-touch RFA than that of tumor-puncturing RFA [6]. Consequently, the cumulative LTPFS rates at 1, 3, and 5 years were 92.4%, 83.4%, and 76.5%, respectively. These results appear comparable or superior to the previously reported outcomes of RFA for CLM [2,3,5].
During no-touch RFA, multiple electrodes are inserted outside the tumor boundary, and centripetal ablation of the tumor is feasible. This type of energy deposition can help obtain a sufficient ablation margin and obliterate feeding and draining vessels in the earlier period of the RFA process. Furthermore, as the electrodes are not in direct contact with the tumor, they may prevent tumor spread around the tumor, and there is no risk of tract seeding. In a recent multicenter prospective study [13], no-touch RFA led to excellent local tumor control after RFA of hepatocellular carcinomas <2.5 cm in size, and the cumulative LTP rate at 2 years was only 1.6%. CLM may also benefit from no-touch RFA, as confirmed in this study.
An interaction effect was found between electrode placement and the T stage of primary CRC on LTPFS. The group with both tumor-puncturing RFA and a T4 stage tumor showed poor LTPFS (hazard ratio, 10.3; 95% CI, 1.2 to 1,345.5; P=0.029) compared to the reference group of no-touch RFA and T1-T3 stages of primary CRC. In addition, in patients undergoing RFA for CLM, the combination of tumor-puncturing RFA and a T4 stage tumor was an independent risk factor for poor LTPFS. Although the association between the T stage of primary CRC and local tumor control of RFA for CLM is not clearly understood, an advanced T stage of primary CRC is known to be associated with poorly differentiated tumors [21]. It has been postulated that an advanced T stage of primary CRC may be associated with aggressive tumor biology of CLM due to poor differentiation, which may yield poor LTPFS after RFA for CLM. Similarly, poorly differentiated hepatocellular carcinoma was identified as a risk factor for LTP after percutaneous RFA [22]. Interestingly, no LTP was found in our study even in patients with T4 stage tumors when no-touch RFA was performed (Fig. 4).
Tumor size and a perivascular or subcapsular location of the CLM are well-known factors affecting local tumor control [1-3,5,23]. Han et al. [2] reported that a tumor size of Ōēź2 cm was associated with LTP after RFA. In addition, perivascular tumors are known to have a relatively increased risk of ablation failure and LTP due to the "heat-sink" effect [24]. Subcapsular tumors are also associated with higher LTP and shorter LTPFS [23,25]. However, tumor size and perivascular or subcapsular location of the tumors were not significantly associated with LTP after RFA in the present study. It is postulated that a sufficient ablative margin obtained by using centripetal RFA or no-touch RFA with multiple electrodes in some cases may have resulted in favorable local tumor control in our study.
It is known that large tumor size (>3 cm) and more than one site of extrahepatic disease are poor prognostic factors for OS [5]. However, no factors were found to be significantly associated with OS in this study. Given that tumors smaller than 3 cm were included in the present study, RFA for CLM <3 cm seemed to lead to good survival outcomes when an aggressive ablation strategy was adopted. In addition, as only three of the 92 patients had extrahepatic disease, it may have been difficult to ascertain statistical significance. Further studies with a larger study population are warranted to verify these results.
The present study had several limitations. First, it was a retrospective study performed at a single medical center. Therefore, selection bias may have occurred. Second, although the ablative margin is known to be a factor associated with LTP, it was not assessed because there is no standardized method for evaluating the ablative margin, and an accurate assessment of the ablative margin is sometimes challenging when the index tumor is not demarcated within the ablation zone. However, the effect of a sufficient ablative margin could be indirectly inferred from the difference in ablation zone size between no-touch RFA and tumor-puncturing RFA. This assumption is supported by a randomized controlled trial between no-touch RFA and conventional tumor-puncturing RFA, in which no-touch RFA provided a larger ablative margin than conventional tumor-puncturing RFA [6]. Third, this study only included patients with a single hepatic metastasis, as we intended to evaluate whether LTPFS and OS after RFA are affected by the specific tumor location through a tumor-based analysis. Fourth, the diagnosis of hepatic metastasis was made based on imaging findings, not a histologic diagnosis, in almost all cases. Although this policy reflects actual clinical practice, patients with a false-positive diagnosis of metastasis may have been included in this cohort.
In conclusion, both LTPFS and OS were promising after percutaneous RFA of a single CLM. LTP was not observed in patients who underwent no-touch RFA. The group with both tumor-puncturing RFA and a T4 stage tumor showed poor LTPFS. No risk factors were identified for poor OS.
NotesAuthor Contributions Conceptualization: Lee HJ, Lee MW, Kang TW. Data acquisition: Lee MW, Rhim H. Data analysis or interpretation: Lee HJ, Lee MW, Ahn SH, Cha DI, Ko SE, Song KD. Drafting of the manuscript: Lee HJ, Lee MW. Critical revision of the manuscript: Lee HJ, Lee MW, Ahn SH, Cha DI, Ko SE, Kang TW, Song KD, Rhim H. Approval of the final version of the manuscript: all authors. References1. Camacho JC, Petre EN, Sofocleous CT. Thermal ablation of metastatic colon cancer to the liver. Semin Intervent Radiol 2019;36:310ŌĆō318.
2. Han K, Kim JH, Yang SG, Park SH, Choi HK, Chun SY, et al. A single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology 2021;298:212ŌĆō218.
3. Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol 2018;29:268ŌĆō275.
4. Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol 2015;25:3438ŌĆō3454.
5. Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes: a 10-year experience at a single center. Radiology 2016;278:601ŌĆō611.
6. Park SJ, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH, et al. Switching monopolar no-touch radiofrequency ablation using octopus electrodes for small hepatocellular carcinoma: a randomized clinical trial. Liver Cancer 2021;10:72ŌĆō81.
7. Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36:166ŌĆō175.
8. Kurilova I, Bendet A, Petre EN, Boas FE, Kaye E, Gonen M, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer 2021;20:e82ŌĆōe95.
9. Hakime A, Yevich S, Tselikas L, Deschamps F, Petrover D, De Baere T. Percutaneous thermal ablation with ultrasound guidance: fusion imaging guidance to improve conspicuity of liver metastasis. Cardiovasc Intervent Radiol 2017;40:721ŌĆō727.
10. Bae JW, Lee MW, Kang TW, Song KD, Cha DI, Min JH, et al. Percutaneous radiofrequency ablation for hepatic metastasis of colorectal cancer: assessment of tumor visibility and the feasibility of the procedure with planning ultrasonography. Ultrasonography 2022;41:189ŌĆō197.
11. Lee DH, Lee JM. Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean J Radiol 2018;19:545ŌĆō559.
12. Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography 2014;33:227ŌĆō239.
13. Lee DH, Lee MW, Kim PN, Lee YJ, Park HS, Lee JM. Outcome of no-touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology 2021;301:229ŌĆō236.
14. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria: a 10-year update. Radiology 2014;273:241ŌĆō260.
15. Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267ŌĆō1274.
16. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology 2016;280:300ŌĆō312.
17. Cha DI, Lee MW, Jeong WK, Ha SY, Ahn SH, Rhim H, et al. Comparison of ablation performance between dual internally cooled wet tip and conventional dual internally cooled tip radiofrequency electrodes: an experimental study in ex vivo bovine liver. Int J Hyperthermia 2021;38:332ŌĆō340.
18. Puijk RS, Ahmed M, Adam A, Arai Y, Arellano R, de Baere T, et al. Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology 2021;301:533ŌĆō540.
19. Calandri M, Yamashita S, Gazzera C, Fonio P, Veltri A, Bustreo S, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol 2018;28:2727ŌĆō2734.
20. Sotirchos VS, Petrovic LM, Gonen M, Klimstra DS, Do RK, Petre EN, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology 2016;280:949ŌĆō959.
21. Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H. Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta Oncol 2010;49:57ŌĆō62.
22. Chinnaratha MA, Sathananthan D, Pateria P, Tse E, MacQuillan G, Mosel L, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol 2015;27:349ŌĆō354.
23. Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol 2003;38:977ŌĆō981.
Flow diagram of patient selection for the study.RFA, radiofrequency ablation; US, ultrasonography.
 Fig.┬Ā1.A 41-year-old man with rectal cancer and a single hepatic metastasis.A. Magnetic resonance image of the axial hepatobiliary phase shows a 1.0-cm hepatic metastasis (arrow) in the liver segment 6 subcapsular area. B. Fusion imaging of real-time ultrasonography and pre-acquired magnetic resonance imaging was used to localize the tumor (arrow). C. Ultrasound image shows two parallel radiofrequency electrodes placed outside the tumor (arrow) using the no-touch radiofrequency ablation technique. D. Immediate axial computed tomography image after radiofrequency ablation shows that the tumor (arrow) is completely covered with a sufficient ablative margin. No local tumor progression was noted during the 11.8-month follow-up period.
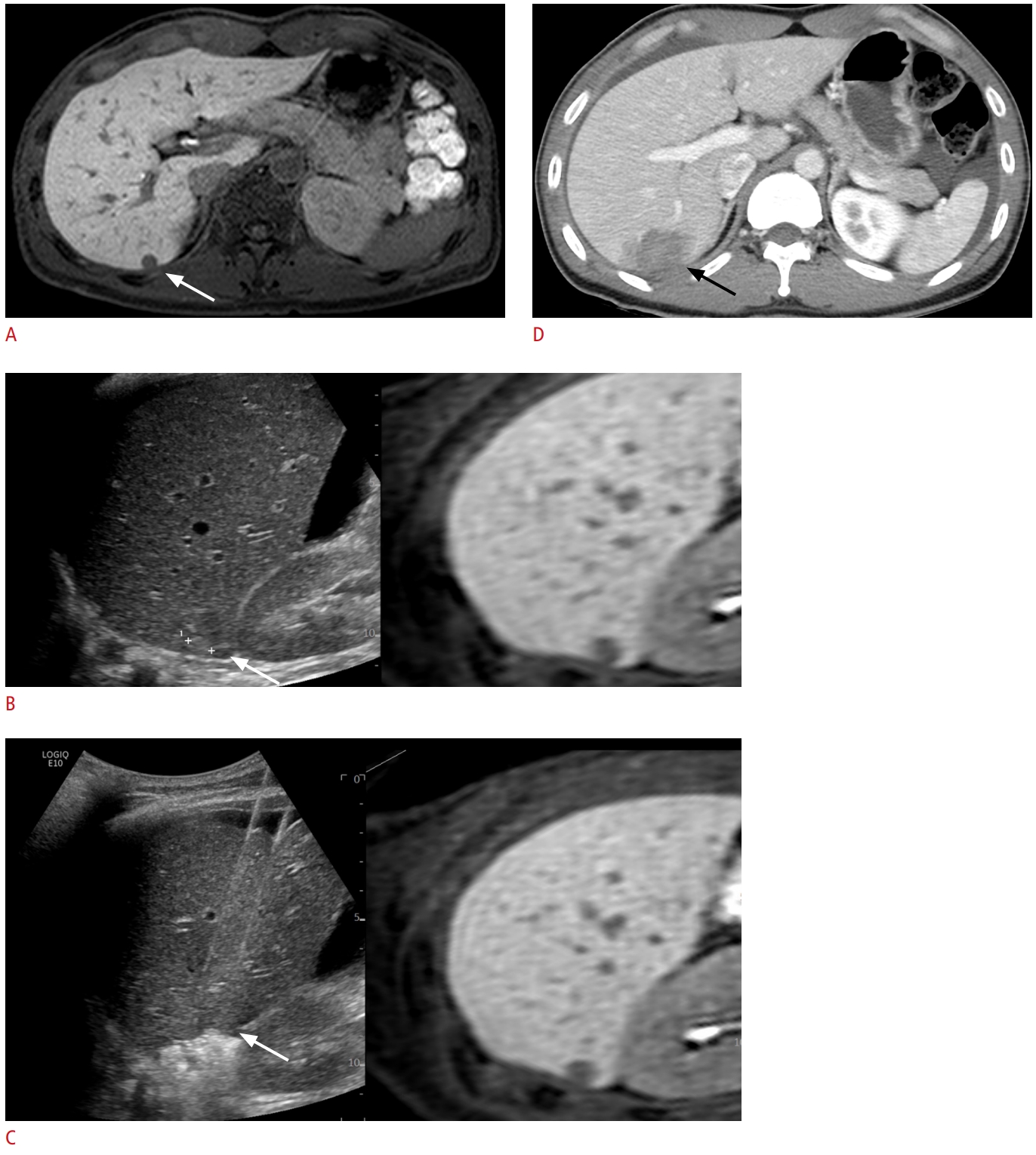 Fig.┬Ā2.A 63-year-old man with rectal cancer and a single hepatic metastasis.A. Axial hepatobiliary phase magnetic resonance image shows a 1.3-cm hepatic metastasis (arrow) in the liver segment 4. B. Fusion imaging of real-time ultrasound and pre-acquired magnetic resonance imaging was used to localize the tumor (arrow). C. Ultrasound image shows radiofrequency electrodes placed through the tumor (arrow), using the tumor-puncturing technique. D. Immediate axial computed tomography image after radiofrequency ablation shows that the tumor (arrow) is completely ablated with sufficient margins. E. Axial arterial phase magnetic resonance image acquired 4.7 months after radiofrequency ablation shows a 1.8-cm peripheral rim enhancing lesion (arrow) adjacent to the ablative margin, suggesting local tumor progression.
 Fig.┬Ā3.Differences in local tumor progression-free survival (LTPFS) according to the radiofrequency ablation technique, the T stage, and the combination thereof.A. LTPFS tended to be higher with no-touch radiofrequency ablation (RFA) than with tumor-puncturing RFA. However, this difference was not statistically significant. B. LTPFS was significantly different between the T1-T3 and T4 stages of primary colorectal cancer. C. LTPFS was significantly different between the combinations of the method of electrode placement and the T stage of primary colorectal cancer.
 Fig.┬Ā4.Differences in overall survival (OS) according to the radiofrequency ablation technique, the T stage, and the combination thereof.A. OS was not significantly different between the tumor-puncturing and no-touch radiofrequency ablation groups. B. OS tended to be higher in T1-T3 than in T4. However, this difference was not statistically significant. C. OS tended to be different according to the combinations of the method of electrode placement and T staging of primary colorectal cancer. However, this difference was not statistically significant.
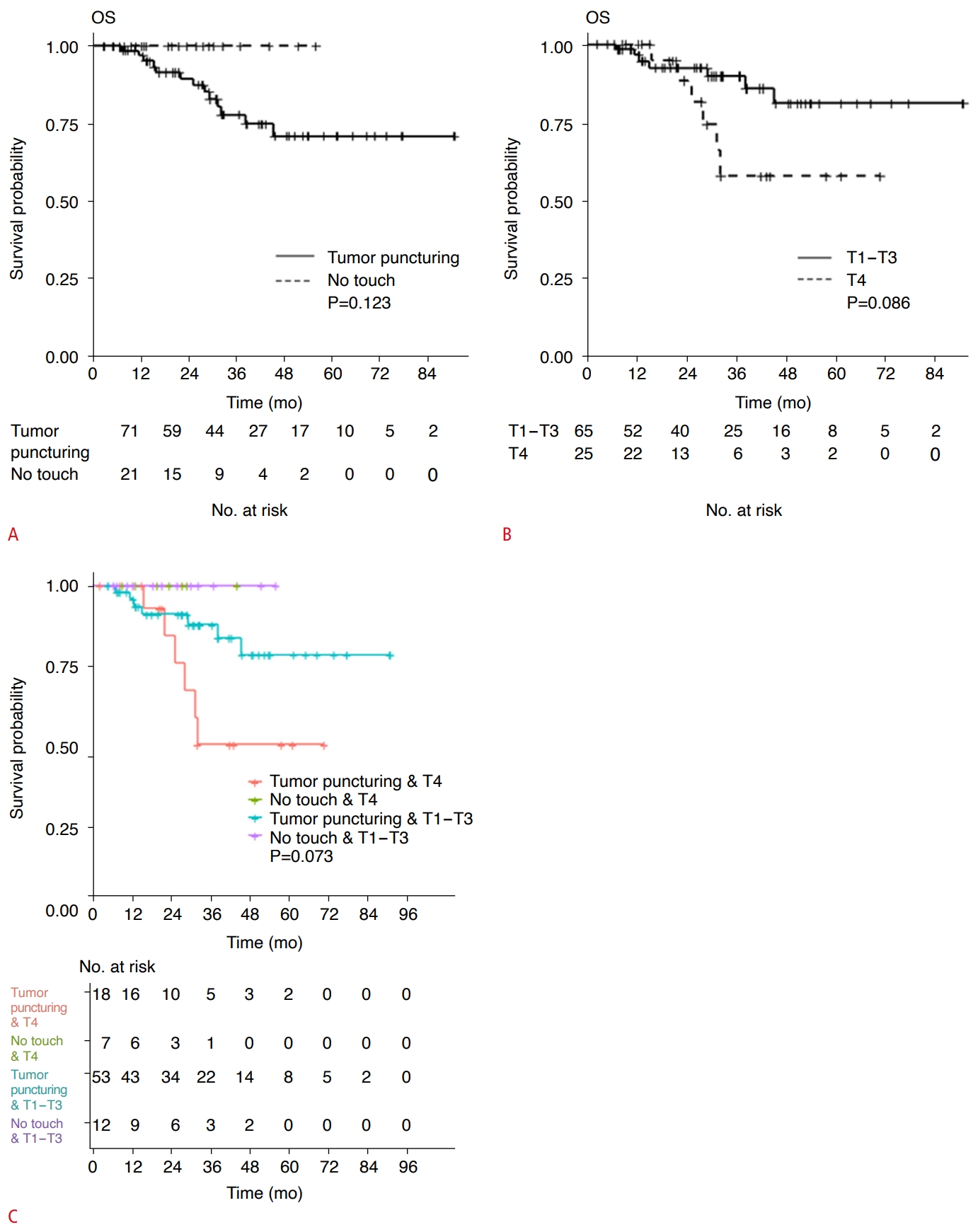 Fig.┬Ā5.Table┬Ā1.Demographic and clinical characteristics of the study patients Table┬Ā2.Ratio of longest diameter and volume between the ablation zone and tumor Table┬Ā3.Univariable and multivariable analyses of the risk factors for LTPFS Hazard ratios and P-values were obtained using Cox regression analysis. The reference category for each categorical variable is indicated by square brackets in the first column. LTPFS, local tumor progression-free survival; CI, confidence interval; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9. Table┬Ā4.Interaction effect between the no-touch versus tumor-puncturing technique and the T stage Table┬Ā5.Univariable analyses of risk factors for OS |