AbstractPurposeThis study investigated the ultrasound (US) features of malignancy in patients with H├╝rthle cell neoplasms (HCNs) of the thyroid gland.
MethodsThe present study included 139 HCNs that had undergone surgical excision at a single institution from 1996 to 2020 and had preoperative US images. The sonographic characteristics of HCNs were correlated with their pathological results. The US findings associated with malignancy were explored using logistic regression analysis, and the diagnostic performance and cutoff were assessed using receiver operating characteristic analysis.
ResultsThe most common US findings of HCNs were a solid content (76.3%), oval to round shape (100%), hypoechogenicity (70.5%), a smooth margin (95.0%), the halo sign (90.6%), and no calcifications (93.5%). HCNs were commonly smaller in pathologic measurements than in US measurements (smaller, same, and greater than US measurements in 60.4%, 21.6%, and 18.0% of HCNs, respectively; P<0.001). On US, malignant nodules were significantly larger than benign nodules (3.4┬▒1.6 cm vs. 2.2┬▒1.2 cm, P<0.001). Multiple logistic regression showed that the US tumor size was an independent predictor of malignancy (P=0.001; odds ratio, 1.730 for a 1-cm increase [95% confidence interval, 1.258 to 2.375]). The best cutoff US tumor size for predicting malignancy was 3.35 cm (sensitivity, 53.1%; specificity, 87.9%).
H├╝rthle cells are large polygonal cells with eosinophilic granular cytoplasm due to dense mitochondrial content, which may also be described as oncocytic or oxyphilic cells [1,2]. They present with a wide range of pathologic entities, including Hashimoto thyroiditis, chronic lymphocytic thyroiditis, adenomatous goiter with H├╝rthle cell metaplasia, and H├╝rthle cell neoplasms (HCNs) [3].
The term "HCN" has been used to describe encapsulated tumors that contain a minimum of 75% H├╝rthle cells. HCNs are classified as benign H├╝rthle cell adenomas (HCAs), malignant H├╝rthle cell carcinomas (HCCs), and HCNs of uncertain malignant behavior (UMB) [2,4-7]. HCAs do not show capsular or vascular invasion, while HCCs demonstrate entire-thickness capsular invasion and/or the presence of vascular invasion by tumor cells. HCNs of UMB are characterized by an equivocal degree of capsular invasion with absent vascular invasion [5].
The clinical behavior of HCNs has been a topic of substantial debate. HCCs have been considered to behave in a more aggressive fashion than other well-differentiated thyroid cancers [8,9]. While these cancers are traditionally grouped with follicular thyroid cancers, they appear to differ clinically, with a higher rate of nodal metastases, a decreased avidity for I-131, and a lower survival rate [9-11]. They present unique genetic, pathologic, and clinical features different from those of follicular neoplasms [11]. Due to the clinical uncertainty associated with these lesions, many surgeons treat them more aggressively than follicular neoplasms [12].
Several prior studies have described the sonographic appearance of HCNs. These reports have documented the typical features of HCNs as solid hypoechoic masses with a characteristic halo sign [13-17]. Fewer investigators have tried to differentiate HCCs from HCAs. There have also been some differences in the methods of collecting study populations. First, Santana et al. analyzed the characteristics of malignancy in pathologically proven HCN cases after lobectomy or thyroidectomy, and concluded that there was no significant distinguishing factor between benignity and malignancy [16]. In contrast, Lee et al. [13] and Kim et al. [18] performed pathologic and radiologic evaluation of pathologically confirmed HCN cases among lesions suspected to be HCNs on fine-needle aspiration (FNA), and reported that tumor size was a single independent predictive factor. FNA alone cannot diagnose HCN, and in this process, H├╝rthle cell-related conditions other than HCN, such as goiter with H├╝rthle cell metaplasia or Hashimoto thyroiditis, can be included [13,18]. Thus, there is a possibility that previous analyses may have contained irrelevant data, such as incidentally found HCNs. Therefore, the population of the present study was selected from pathologically proven HCN cases that could be correlated with preoperative ultrasound (US) findings. Furthermore, due to the diseaseŌĆÖs rarity, previous studies have included only small case numbers or a mixed population of patients with other etiologies of malignancy, which may have limited the applicability of their statistical analyses [13-16,19]. The primary objective of this study was to evaluate the US features that suggest malignancy in HCNs. To the authorsŌĆÖ knowledge, this is the largest study to date that has investigated various US features based on surgically proven cases.
This retrospective study was approved by the institutional review board of our institution (Samsung Medical Center 2021-05-009), and informed consent was obtained from patients before they underwent the biopsy procedures.
The study was conducted in patients with HCN (including HCA and HCC) according to surgical reports obtained from the authorsŌĆÖ institution between January 1996 and November 2020 (Fig. 1). A group of 188 patients was identified from this period of approximately 24 years. Among them, 157 lesions from 153 patients were included in the study after the exclusion of 35 patients who did not consent to the provision of personal information.
Furthermore, 18 patients were excluded due to difficulties in the radiologic-pathologic correlation resulting from the presence of multiple tumors or the absence of available US images. Finally, 139 cases in 135 HCN patients (31 men and 104 women; mean age┬▒standard deviation, 52.2┬▒12.8 years) who underwent surgical resection were enrolled in the study.
US was performed by one of 10 radiologists using high-resolution US equipment with 5-12 MHz linear array transducers. The entire thyroid gland and lateral neck compartments were analyzed throughout the examinations. The obtained images were retrospectively analyzed by two radiologists with 22 years and 3 years of experience, respectively.
In all cases, the following nine US characteristics and Korean-Thyroid Imaging Reporting and Data System (K-TIRADS) categories of the lesions were evaluated: tumor size, internal content, echogenicity, margin, orientation, shape, internal calcification, presence of a halo sign, and vascularity [20].
Tumor size was recorded as the longest diameter measured. The internal content of the mass was assessed as solid, predominantly solid (cystic portion Ōēż50%), predominantly cystic solid (cystic portion >50%), or cystic. The echogenicity was assessed in relation to the normal thyroid parenchyma and strap muscle. The margin of the mass was classified as smooth, spiculated/microlobulated, or ill-defined. The orientation was divided into parallel or nonparallel depending on whether the anteroposterior diameter was larger or smaller than the transverse or longitudinal diameter. Shapes were classified as irregular if the mass was neither round nor oval. Internal calcification was analyzed as follows: no calcification, microcalcification (echogenic foci Ōēż1 mm), macrocalcification (echogenic foci >1 mm), and rim calcification (peripheral curvilinear echogenic rim). The presence of a hypoechoic rim (halo sign) was also assessed. Lastly, the tumorŌĆÖs vascularity was classified as marked intranodular, mild intranodular, perinodular, none, or undetermined.
The target nodules had undergone at least one US-guided FNA or core needle biopsy (CNB) preoperatively. The first routine test for a thyroid nodule is typically FNA, but CNB was often carried out in patients with equivocal or nondiagnostic FNA results. Some nodules also underwent neither FNA nor CNB due to the presence of another primary tumor that required treatment, such as papillary thyroid carcinoma.
In FNA, the transducer was positioned vertically directly above the lesion, and a 23-gauge needle targeted the center of the lesion and was advanced using the freehand technique. While continuously verifying whether the needle tip was inside the lesion, mild negative pressure was applied to the syringe for effective aspiration. FNA was usually performed 1-2 times for each nodule at the authorsŌĆÖ institution. When a sufficient sample was obtained, it was smeared on a slide glass, fixed in 95% ethyl alcohol, and sent to the pathology department.
For CNB, an automatic biopsy system was used. After administering local anesthesia with lidocaine at the puncture site, the transducer was placed longitudinally so that the entire tract of the biopsy gun could be traced on US. Samples were obtained at least twice so that the lesion, the capsule of the lesion, and the normal thyroid parenchyma were all included [21]. At least two specimen cores were fixed in formalin and sent to the pathology department.
The pathologic diagnosis of HCNs was made by one of nine pathologists following World Health Organization criteria, followed by further division into three categories: HCA, HCC, and HCN of UMB [2,5]. The degrees of capsular invasion and vascular invasion were used to classify the specimens into these subtypes. The sizes and locations of the tumors were recorded. The tumor size was recorded based on the largest size relative to the long axis. Postoperative pathologic reports and pathologic tumor sizes were retrospectively analyzed to validate their concordance with the measurements made on US.
The US characteristics of the lesions were assessed according to the pathologic subtypes. The significance of the difference between the US-measured tumor size and the pathologic tumor size measured using the surgical specimen was assessed. The Shapiro-Wilk test was conducted to verify the normality assumption. If the normality assumption was satisfied, the paired t-test was performed; if not, the Wilcoxon signed rank test was used. For cases of surgically confirmed HCN, the Pearson chi-square test was carried out to determine whether there was a significant difference in the distribution of lesions of Bethesda category 4 or higher as surgical candidates in the CNB and FNA groups. Logistic regression analysis was conducted to explore the association between clinical and US features and the benign and malignant nature of HCNs. All factors with P<0.05 in the univariate analysis were included in multiple logistic regression. A receiver operating characteristics curve analysis was conducted, and the area under the curve was estimated to evaluate the ability of US tumor size to predict malignancy. The best cutoff for US tumor size to distinguish HCCs from HCAs was determined.
Of the 139 HCNs, the final pathology examinations found 99 (71.2%) HCAs, 32 (23.0%) HCCs, and eight (5.8%) HCNs of UMB. The clinical and radiologic features of HCN along with their final pathology results are presented in Table 1. On US, HCNs commonly presented as solid (76.3%), oval to round (100%), hypoechoic (70.5%) masses, with a smooth margin (95.0%) and a halo sign (90.6%), as well as an absence of internal calcification (93.5%). HCCs were significantly larger than HCAs (mean┬▒standard deviation, 3.4┬▒1.6 cm vs. 2.2┬▒1.2 cm; P<0.001).
Table 2 shows the distribution of HCAs, HCCs, and HCNs of UMB according to the test modality (FNA/CNB) and Bethesda categories. Most of the cases belonged to Bethesda III or IV, while only six of 119 cases (5.0%) belonged to Bethesda V or VI when excluding cases that did not undergo biopsy due to any other coexisting malignancy. There were also no cases diagnosed as Bethesda V or VI in masses that underwent CNB.
The univariate analysis, as shown in Table 3, showed that three features were significantly associated with HCA versus HCC: internal content (P=0.019), US tumor size (P<0.001), and pathologic tumor size (P<0.001). HCC masses more frequently showed cystic changes than HCAs (38%, 12/32 vs. 17%, 17/99, respectively; P<0.001) (Fig. 2). Other factors such as echogenicity, margin, orientation, calcification, the halo sign, vascularity, K-TIRADS category, and Bethesda category did not show statistical significance for differentiating HCC from HCA. The multiple logistic regression analysis, as presented in Table 4, revealed that the US tumor size was an independent significant predictor of malignancy (P=0.001; odds ratio, 1.730 for each 1-cm increase [95% confidence interval, 1.258 to 2.375]) (Fig. 3). The best cutoff value of US tumor size for predicting malignancy was 3.35 cm (sensitivity, 53.1%; specificity, 87.9%). Table 5 presents the diagnostic performance according to variable US tumor size cutoff values for predicting malignant HCNs (HCCs).
HCNs were commonly smaller when pathologically measured using surgical specimens than when measured by US (smaller, same, and greater than US measurements in 60.4%, 21.6%, and 18.0% of cases, respectively; P<0.001). Furthermore, 12% of HCNs showed a more than 30% smaller size after surgery compared to the preoperative tumor size. There was no significant difference in the magnitude of the size discrepancy between the HCC group and the HCA group.
Previous studies have reported that 5% to 35% of HCNs are malignant [22-24]. In the present study, 23% of all HCN cases were identified as malignancies. Although timely surgical treatment is essential, it remains challenging to reliably distinguish malignant from benign HCNs in preoperative settings [3]. For this reason, surgeons may have difficulty deciding when to operate on a thyroid nodule suspected of HCN on FNA or CNB, and whether to perform lobectomy or total thyroidectomy.
Researchers have investigated factors that can predict the malignant potential of HCNs preoperatively to aid the surgeonŌĆÖs clinical decision process, including the extent of surgery. In particular, there have been many studies of clinical factors such as age, sex, and tumor size; malignant potential increases in male patients who are older and have a larger tumor size [18,25].
However, it is difficult to predict HCC even with the current K-TIRADS. Factors such as echogenicity, margin, and internal calcification are known to be insignificant for predicting malignant potential in HCNs [18]. Tumor size is the most significant factor in predicting malignant potential, and its cutoff was reported to range from 1.5 to 4.0 cm [13,17,18,26]. In this study, US tumor size was found to be a significant predictor of malignancy in HCNs, with a preoperative US tumor size >3.35 cm as the best cutoff to distinguish malignant from benign HCNs. Each US and pathologic tumor size used to predict malignancy showed meaningful results in the univariate analysis. However, as the pathologic tumor size cannot be known before surgery, the US tumor size, which can be obtained preliminarily, is more useful for preoperative planning.
To the best of the authorsŌĆÖ knowledge, this is the first study to identify the possibility that cystic changes detected on US may be a significant factor in predicting the malignancy of HCNs using univariate regression analysis. As the solidity of nodules decreases, the degree of suspicion for malignancy also becomes lower [27]. However, HCNs did not follow this theory in the present study, which may be due to the fact that carcinomas are generally larger than adenomas and thus have a higher probability of hyaline degeneration or internal hemorrhage/infarction. Due to the small number of HCNs with cystic changes, statistical significance was not reached in the multivariate analysis.
In this study, cystic changes on US in HCNs were shown to be related to histology. These changes often lead to size discrepancies between US and pathology. No significant difference was found in the magnitude of the size reduction between the HCC group and the HCA group. Several studies have reported that thyroid nodules (including HCN) may undergo necrosis or infarction after traumatic procedures, such as FNA or CNB [28,29]. Microvascular supply inhibition or vascular supply compromise due to tissue loss and the intrinsic energy deficiency of oncocytic cells have been suggested as causes, and the size of tumors may decrease through hemorrhage and fibrosis [29-31].
This study had several limitations. First, this was a retrospective study. However, due to the rarity of HCNs, a retrospective approach to surgically confirmed cases was unavoidable. Second, the findings were not compared between HCN and other histologic categories. Third, the data was collected over a 20-year period, for which reason the US images may have been heterogeneous.
In conclusion, US tumor size was identified as an independent predictor of malignancy in HCNs, and a US tumor size >3.35 cm might be used as a criterion to suggest malignancy. The size of HCNs often showed discrepancies between US and pathologic measurements.
NotesAuthor Contributions Conceptualization: Kim MJ, Shin JH, Hahn SY, Oh YL, Kim SW, Kim TH, Lim Y, Lee S. Data acquisition: Kim MJ, Shin JH, Hahn SY, Oh YL, Kim SW, Kim TH, Lim Y, Lee S. Data analysis or interpretation: Kim MJ, Shin JH, Hahn SY, Oh YL, Kim SW, Kim TH, Lim Y, Lee S. Drafting of the manuscript: Kim MJ, Shin JH, Hahn SY, Oh YL, Kim SW, Kim TH, Lim Y, Lee S. Critical revision of the manuscript: Kim MJ, Shin JH, Hahn SY, Oh YL, Kim SW, Kim TH, Lim Y, Lee S. Approval of the final version of the manuscript: all authors. References2. Kakudo K, Bychkov A, Bai Y, Li Y, Liu Z, Jung CK. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int 2018;68:641ŌĆō664.
3. Elliott DD, Pitman MB, Bloom L, Faquin WC. Fine-needle aspiration biopsy of Hurthle cell lesions of the thyroid gland: a cytomorphologic study of 139 cases with statistical analysis. Cancer 2006;108:102ŌĆō109.
4. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer 1989;63:908ŌĆō911.
5. Erickson LA, Jin L, Goellner JR, Lohse C, Pankratz VS, Zukerberg LR, et al. Pathologic features, proliferative activity, and cyclin D1 expression in Hurthle cell neoplasms of the thyroid. Mod Pathol 2000;13:186ŌĆō192.
6. Bai Y, Kakudo K, Jung CK. Updates in the pathologic classification of thyroid neoplasms: a review of the World Health Organization classification. Endocrinol Metab (Seoul) 2020;35:696ŌĆō715.
7. Shawky M, Sakr M. Hurthle cell lesion: controversies, challenges, and debates. Indian J Surg 2016;78:41ŌĆō48.
8. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U. S S;S:SŌĆōS.
9. Lopez-Penabad L, Chiu AC, Hoff AO, Schultz P, Gaztambide S, Ordonez NG, et al. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer 2003;97:1186ŌĆō1194.
10. Kushchayeva Y, Duh QY, Kebebew E, Clark OH. Prognostic indications for Hurthle cell cancer. World J Surg 2004;28:1266ŌĆō1270.
11. Maxwell EL, Palme CE, Freeman J. Hurthle cell tumors: applying molecular markers to define a new management algorithm. Arch Otolaryngol Head Neck Surg 2006;132:54ŌĆō58.
12. Pisanu A, Sias L, Uccheddu A. Factors predicting malignancy of Hurthle cell tumors of the thyroid: influence on surgical treatment. World J Surg 2004;28:761ŌĆō765.
13. Lee KH, Shin JH, Ko ES, Hahn SY, Kim JS, Kim JH, et al. Predictive factors of malignancy in patients with cytologically suspicious for Hurthle cell neoplasm of thyroid nodules. Int J Surg 2013;11:898ŌĆō902.
14. Li P, Liu P, Zhang H. Ultrasonic diagnosis for thyroid Hurthle cell tumor. Cancer Biomark 2017;20:235ŌĆō240.
15. Maizlin ZV, Wiseman SM, Vora P, Kirby JM, Mason AC, Filipenko D, et al. Hurthle cell neoplasms of the thyroid: sonographic appearance and histologic characteristics. J Ultrasound Med 2008;27:751ŌĆō757.
16. Santana NO, Freitas RM, Marcos VN, Chammas MC, Camargo RY, Schmerling CK, et al. Diagnostic performance of thyroid ultrasound in Hurthle cell carcinomas. Arch Endocrinol Metab 2019;63:300ŌĆō305.
17. Lee SK, Rho BH, Woo SK. Hurthle cell neoplasm: correlation of gray-scale and power Doppler sonographic findings with gross pathology. J Clin Ultrasound 2010;38:169ŌĆō176.
18. Kim TH, Lim JA, Ahn HY, Lee EK, Min HS, Won Kim K, et al. Tumor size and age predict the risk of malignancy in Hurthle cell neoplasm of the thyroid and can therefore guide the extent of initial thyroid surgery. Thyroid 2010;20:1229ŌĆō1234.
19. Rago T, Di Coscio G, Basolo F, Scutari M, Elisei R, Berti P, et al. Combined clinical, thyroid ultrasound and cytological features help to predict thyroid malignancy in follicular and Hupsilonrthle cell thyroid lesions: results from a series of 505 consecutive patients. Clin Endocrinol (Oxf) 2007;66:13ŌĆō20.
20. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol 2016;17:370ŌĆō395.
21. Han S, Shin JH, Hahn SY, Oh YL. Modified core biopsy technique to increase diagnostic yields for well-circumscribed indeterminate thyroid nodules: a retrospective analysis. AJNR Am J Neuroradiol 2016;37:1155ŌĆō1159.
22. Melck A, Bugis S, Baliski C, Irvine R, Anderson DW, Wilkins G, et al. Hemithyroidectomy: the preferred initial surgical approach for management of Hurthle cell neoplasm. Am J Surg 2006;191:593ŌĆō597.
23. Carcangiu ML, Bianchi S, Savino D, Voynick IM, Rosai J. Follicular Hurthle cell tumors of the thyroid gland. Cancer 1991;68:1944ŌĆō1953.
24. McIvor NP, Freeman JL, Rosen I, Bedard YC. Value of fine-needle aspiration in the diagnosis of Hurthle cell neoplasms. Head Neck 1993;15:335ŌĆō341.
25. Strazisar B, Petric R, Sesek M, Zgajnar J, Hocevar M, Besic N. Predictive factors of carcinoma in 279 patients with Hurthle cell neoplasm of the thyroid gland. J Surg Oncol 2010;101:582ŌĆō586.
26. Dahl LD, Myssiorek D, Heller KS. Hurthle cell neoplasms of the thyroid. Laryngoscope 2002;112:2178ŌĆō2180.
27. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid Imaging Reporting and Data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid 2016;26:562ŌĆō572.
28. Keyhani-Rofagha S, Kooner DS, Keyhani M, O'Toole RV. Necrosis of a Hurthle cell tumor of the thyroid following fine needle aspiration: case report and literature review. Acta Cytol 1990;34:805ŌĆō808.
29. Layfield LJ, Lones MA. Necrosis in thyroid nodules after fine needle aspiration biopsy: report of two cases. Acta Cytol 1991;35:427ŌĆō430.
Flow chart of the study population.HCC, H├╝rthle cell carcinoma; HCA, H├╝rthle cell adenoma; HCN of UMB, H├╝rthle cell neoplasm of uncertain malignant behavior.
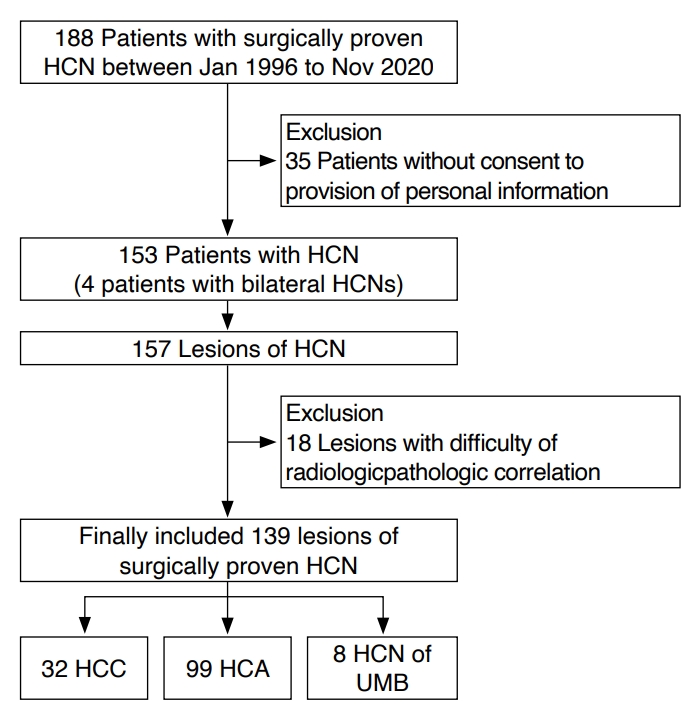 Fig.┬Ā1.A 71-year-old woman with H├╝rthle cell carcinoma in the left thyroid lobe.Transverse (A) and longitudinal (B) ultrasonography show a 3.8-cm predominantly cystic mass (crosses) with a hypoechoic, smooth margin, a parallel orientation, an oval shape, no microcalcification, and a halo sign. The mass was initially classified as low-suspicion (K-TIRADS 3) on ultrasonography. The core needle biopsy suggested H├╝rthle cell neoplasm, and left thyroid lobectomy revealed H├╝rthle cell carcinoma with capsular invasion. K-TIRADS, Korean Thyroid Imaging Reporting and Data System.
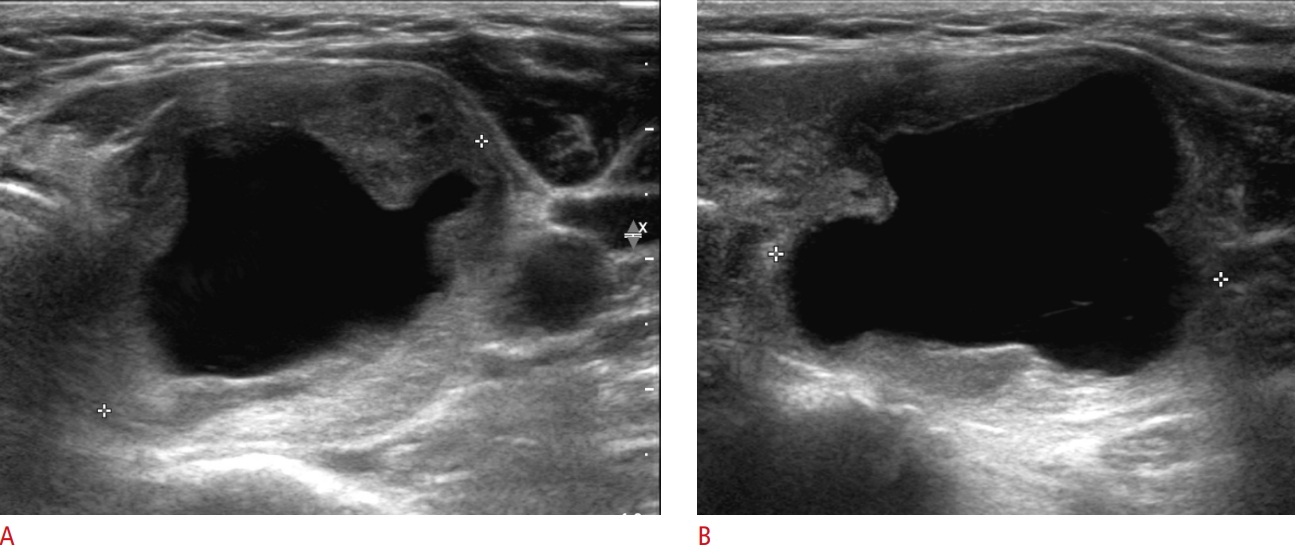 Fig.┬Ā2.A 63-year-old woman with H├╝rthle cell adenoma in the right thyroid lobe.Longitudinal (A) and color (B) ultrasonography reveals a 2.1-cm solid mass (crosses) with a hypoechoic, smooth margin, a parallel orientation, an oval shape, no microcalcification, a halo sign, and markedly increased vascularity. The mass was initially classified as low-suspicion (K-TIRADS 4). Based on the core needle biopsy, follicular neoplasm was suspected, and right thyroid lobectomy revealed H├╝rthle cell adenoma. K-TIRADS, Korean Thyroid Imaging Reporting and Data System.
 Fig.┬Ā3.Table┬Ā1.The clinical and radiologic characteristics of HCNs according to the final pathology results
Table┬Ā2.Bethesda categories by FNA or CNB in 119 HCNs biopsied Table┬Ā3.Univariate logistic regression analysis regarding the distinction of HCC from HCA |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




