AbstractPurposeThe aim of this multicenter study was to investigate the malignancy risk of minimally cystic thyroid nodules (MCTNs) using cyto-histopathologic diagnoses as the reference standard.
MethodsFrom June 2015 to September 2015, 5,601 thyroid nodules (Ōēź1 cm) from 4,989 consecutive patients who underwent thyroid ultrasonography (US) at 26 institutions were retrospectively analyzed. Each thyroid nodule was categorized according to its cystic proportion: purely solid, minimally cystic (Ōēż10%), and partially cystic (>10%). The malignancy risk of MCTNs was compared with those of purely solid nodules and partially cystic thyroid nodules (PCTNs). The malignancy risk of MCTNs was assessed according to echogenicity and the presence of suspicious US features.
ResultsThe prevalence of MCTNs was 22.5%. The overall malignancy risk of MCTNs was 8.8%, which was significantly lower than that of purely solid nodules (29.5%) (P<0.001), and slightly higher than that of PCTNs (6.2%) (P=0.013). The risk of malignancy associated with MCTNs was similar to that of PCTNs regardless of echogenicity or the presence of suspicious US features (all P>0.05). MCTNs were associated with a higher risk of malignancy in hypoechoic nodules than in isohyperechoic nodules and in nodules with suspicious US features than in those without suspicious US features (all P<0.001).
Ultrasonography (US) is the primary tool used to evaluate thyroid nodules and their malignancy risk, and to identify patients who require fine-needle aspiration (FNA) or core needle biopsy (CNB) [1]. Many international societies have proposed risk stratification systems (RSSs) for the clinical management of thyroid nodules. Most of these systems stratify malignancy risk based on US features such as composition, echogenicity, punctate echogenic foci (microcalcifications), nonparallel orientation (taller-than-wide shape), and irregular (microlobulated/spiculated) margin [2-7].
Although US-based RSSs have been increasingly used for the diagnosis and management of thyroid nodules [2-7], each RSS incorporates different definitions for the US lexicon that describes solid composition [8]. The 2021 Korean Thyroid Imaging Reporting and Data System (K-TIRADS) [5] defines solid nodules as those for which an obvious cystic component is not visualized, whereas the American College of Radiology (ACR)-TIRADS [6] defines solid nodules as nodules that contain small cystic components occupying no more than approximately 5% of the overall volume. The European (EU)-TIRADS [7] defines solid nodules as nodules composed almost entirely of soft tissue with <10% liquid. The American Thyroid Association (ATA) [3] and American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi (AACE/ACE/AME) do not clearly specify definitions of composition [4]. These differences in definition could affect the overall diagnostic performance of US-based RSSs. Thus, appropriate US-based definitions for solid composition need to be standardized for thyroid nodules by assessing the malignancy risk of minimally cystic thyroid nodules (MCTNs).
A previous study [9] that evaluated the malignancy risk of MCTNs defined MCTNs as nodules with a cystic portion Ōēż10% compared with purely solid or partially cystic thyroid nodules (PCTNs). MCTNs showed a low risk of malignancy (3.3%), similar to PCTNs, regardless of echogenicity or the presence of suspicious US features [9]. To the best of the authorsŌĆÖ knowledge, however, no multicenter study has reported the malignancy risk of MCTNs. Therefore, the aim of this multicenter study was to investigate the malignancy risk of MCTNs using cyto-histopathologic diagnoses.
The institutional review boards of 26 different hospitals approved this study, and the requirement for informed patient consent was waived because of its retrospective nature.
Patient data were collected from the 26 hospitals (Thyroid Imaging Network Korea, THINK). Consecutive patients who underwent thyroid US between June 2015 and September 2015 were enrolled in this study: Patients who had nodules Ōēź1 cm and had undergone FNA, CNB, or surgery for nodules were included. Patients were excluded from the study if the thyroid nodule was smaller than 1 cm, there was no reference standard test (biopsy or surgery), or the image quality was suboptimal. Among 22,775 consecutive patients who had undergone thyroid US at 26 institutions, 16,679 patients were excluded due to a thyroid nodule size less than 1 cm (n=12,130), no reference standard test (biopsy or surgery) (n=4,304), or suboptimal image quality (n=245). Among them, 1,015 patients with 1,102 nodules were further excluded because of inconclusive biopsy results. Furthermore, 107 nodules were excluded because the US characteristics could not be analyzed in 59 isolated macrocalcifications (entirely calcified nodules) and 48 purely cystic nodules (Fig. 1).
Ultimately, 5,601 thyroid nodules in 4,989 consecutive patients (4,101 women, 888 men; mean age, 53.3┬▒12.7 years; age range, 19 to 93 years) were included. Among the 5,601 nodules, 1,089 were finally diagnosed as malignant based on histopathological results after surgery (n=927, 85.1%) or malignant FNA or CNB diagnoses (n=162, 14.9%). The other 4,512 nodules were finally diagnosed as benign nodules based on cyto-histopathology after surgery (n=390, 8.6%), at least two benign diagnoses via FNA or CNB (n=594, 13.2%), or one benign diagnosis based on FNA or CNB that did not show follicular neoplasm, suspicious malignant, or malignant biopsy results in the initial or repeat FNA or CNB (n=3,528, 78.2%).
Real-time US was performed with a high-resolution ultrasound scanner that was equipped with a 5ŌĆō12 MHz or an 8ŌĆō15 MHz linear probe. The scanning protocol included both transverse and longitudinal images of the thyroid nodules, using representative Digital Imaging and Communications in Medicine (DICOM) images. To establish a baseline consensus regarding the US lexicon of thyroid nodules, two consensus meetings were held prior to this study. Seventeen experienced radiologists (with 8ŌĆō22 years of experience with thyroid US) participated to reach a consensus on the definitions of the US features to be analyzed.
In this study, US images were analyzed in DICOM format using an online program (AIM AiCRO; https://study.aim-aicro.com). Seventeen experienced radiologists, who were blinded to histopathologic findings and the final diagnoses, retrospectively analyzed the US features of each thyroid nodule according to the revised 2021 K-TIRADS lexicon for composition, echogenicity, orientation (shape), margin, and echogenic foci (calcifications) [5]. The composition of each thyroid nodule was defined according to the percentage of the cystic portion in the entire nodule: purely solid (no cystic portion), MCTNs (cystic portion Ōēż10%), and PCTNs (predominantly cystic or predominantly solid nodules with any cystic portion >10%) (Fig. 2). Vessels, marked hypoechogenicity of the solid portion, fibrosis, or shadowing artifacts were carefully distinguished from minimally cystic changes. Nodule echogenicity was categorized into hypoechogenicity (marked or mild hypoechogenicity) or isohyperechogenicity based on the relative echogenicity of the nodule compared with the normal thyroid parenchyma and the anterior neck muscles (Fig. 3). Punctate echogenic foci (microcalcifications), nonparallel orientation (taller-than-wide shape), and irregular (microlobulated/spiculated) margin were defined as suspicious US features [5,10].
The malignancy risk of MCTNs was evaluated in all nodules and subgroups based on composition, size, echogenicity and the presence of suspicious US features (punctate echogenic foci, nonparallel orientation, and irregular margin). The chi-square test or Fisher exact test was used to compare malignancy risk according to nodule composition in all nodules and according to echogenicity and the presence of suspicious US features in subgroups. All statistical analyses were conducted using SPSS for Windows (version 24.0, IBM Corp., Armonk, NY, USA). A P-value <0.05 was considered to indicate statistical significance.
The demographic characteristics of 5,601 nodules in 4,989 patients are presented in Table 1. The final diagnoses of the malignant nodules included 989 papillary thyroid carcinomas (90.8%) and 100 other malignant tumors (9.2%), which comprised 62 follicular carcinomas (5.7%), 12 medullary carcinomas (1.1%), seven poorly differentiated carcinomas (0.6%), six anaplastic carcinomas (0.6%), five metastases (0.5%), four unspecified malignancies (0.4%), three lymphomas (0.3%), and one squamous cell carcinoma (0.1%).
Table 2 lists the frequency and malignancy risk of 5,601 thyroid nodules according to their composition. The frequency of purely solid nodules, MCTNs, and PCTNs was 3,041 (54.3%), 1,259 (22.5%), and 1,301 (23.2%), respectively. MCTNs were more prevalent in benign nodules than malignant nodules (P<0.001). The overall malignancy risk of MCTNs was 8.8%, which was significantly lower than that of purely solid nodules (29.5%) (P<0.001) and slightly higher than that of PCTNs (6.2%) (P=0.013).
Table 3 shows the frequency and malignancy risk of thyroid nodules according to their composition and echogenicity. MCTNs were found in 12.4% (249/2,014) of the hypoechoic nodule group and 28.2% (1,010/3,587) of the isohyperechoic nodule group. MCTNs were more prevalent in benign nodules than in malignant nodules in both hypoechoic nodules and isohyperechoic nodules (P<0.001 and P=0.004, respectively). MCTNs were associated with a higher malignancy risk in the hypoechoic nodule group (18.1%) than in the isohyperechoic nodule group (6.5%) (P<0.001). However, in both the hypoechoic and isohyperechoic nodule groups, the malignancy risks of MCTNs were similar to those of PCTNs (P=0.203 and P=0.092, respectively) and significantly lower than those of purely solid nodules (all P<0.001).
Table 4 presents the frequency and malignancy risk of thyroid nodules according to their composition and the presence of suspicious US features. MCTNs constituted 16.0% (242/1,514) of the group of nodules with suspicious US features and 24.9% (1,017/4,087) of the group of nodules without suspicious US features. MCTNs were more prevalent among benign nodules than among malignant nodules, both in the group of nodules with suspicious US features and in the group of nodules without suspicious US features (all P<0.001). MCTNs were associated with a higher malignancy risk in nodules with suspicious US features (20.7%) than in those without suspicious US features (6.0%) (P<0.001). However, in both nodules with and without suspicious US features, the malignancy risks of MCTNs were similar to those of PCTNs (P=0.272 and P=0.118, respectively) and significantly lower than those of purely solid nodules (all P<0.001).
Purely solid nodules showed a higher malignancy risk in small nodules (Ōēż2 cm) than in large nodules (>2 cm) (P<0.001). In comparison, MCTNs and PCTNs did not show different malignancy risks according to nodule size (P=0.074 and P=0.141, respectively). In both small (Ōēż2 cm) and large (>2 cm) nodules, the malignancy risks of MCTNs were similar to those of PCTNs (P=0.093 and P=0.056, respectively) and significantly lower than those of purely solid nodules (all P<0.001) (Table 5).
This multicenter study investigated the malignancy risk of thyroid nodules with minimal cystic changes. The distribution of purely solid nodules, MCTNs, and PCTNs was 3,041 (54.3%), 1,259 (22.5%), and 1,301 (23.2%), respectively. The overall malignancy risk of MCTNs was 8.8%, which was significantly lower than that of purely solid nodules (29.5%) (P<0.001). The overall risk of malignancy associated with MCTNs was 8.8%, which was higher than the previously reported result of 3.3%. A previous study [9] reported that the malignancy risk associated with purely solid nodules, MCTNs, and PCTNs was 14.8% (108/730), 3.3% (2/61), and 3.3% (7/209), respectively, with MCTNs showing the same malignancy risk as PCTNs. This difference might be due to the low prevalence of MCTNs (6.1%), few cases of hypoechoic MCTNs (n=9) and MCTNs with suspicious US features (n=6) in the previous study. In this study, MCTNs were more prevalent (22.5%) and there were many cases of hypoechoic MCTNs (n=249) and MCTNs with suspicious US features (n=242). The malignancy risks associated with hypoechoic MCTNs (18.1%) and MCTNs with suspicious US features (20.7%) were higher than those of isohyperechoic MCTNs (6.5%) and MCTNs without suspicious US features (6.0%), which might explain why the overall malignancy risk of MCTNs was higher in the present study than in the previous one [9].
Therefore, it seems appropriate to define solid nodules as purely solid nodules without obvious cystic components in the US lexicon, as this definition enables more accurate risk stratification and higher interobserver agreement. First, the malignancy risk of MCTNs was significantly lower than that of purely solid nodules. Second, the malignancy risk of MCTNs was similar to that of PCTNs in all subgroups categorized according to echogenicity or presence of suspicious US features. Although the overall malignancy risk was slightly higher in MCTNs than PCTNs, the malignancy risk estimated by US features is not based on a single US predictor, but rather a combination of US features [5,11-13].
Finally, a quantitative analysis of the cystic portion may not be accurate because solid nodules present as a continuum [6]. Estimating the cystic portion as <10% or <5% in a nodule is highly subjective, and high interobserver agreement might not be achievable [6,7]. Thus, defining solid nodules as purely solid nodules may help increase the interobserver agreement for solid composition. Moreover, in EU-TIRADS and ACR-TIRADS, minimally cystic nodules are classified as solid, so classifying minimally cystic nodules as partially cystic nodules would be expected to increase the accuracy of the RSS for thyroid nodules and reduce the unnecessary biopsy rate.
Previous investigators reported that most malignant thyroid tumors were solid (81.6%ŌĆō93%) [5,14-17], and the malignancy risk of solid nodules was higher (24.1%ŌĆō34.7%) than that of PCTNs (3.3%ŌĆō7.1%) [12-15]. This study showed similar results, as most of the malignant thyroid tumors were purely solid (82.4%), and solid tumors were associated with higher malignancy risk (29.5%) than PCTNs (6.2%) or MCTNs (8.8%). Accurately determining the presence of minimally cystic changes can be difficult in cases that include complicated cysts with hemorrhage or thick colloid materials, as well as in cases of fibrosis with marked hypoechogenicity [9,17]. In this study, nodules without obvious anechoic cystic change were categorized as purely solid nodules to enhance interobserver agreement.
This study has several limitations. First, cases without a final diagnosis or inconclusive biopsy results were excluded, which may have resulted in selection bias. Second, the retrospective assessment of static sonograms inherently limited the accuracy of the US interpretation for minimally cystic changes. Third, US features were described by different radiologists, resulting in interobserver variability; however, interobserver variability was not investigated in the present study.
The malignancy risk of MCTNs was low (8.8%) and significantly lower than that of purely solid nodules (29.5%). The risk of malignancy associated with MCTNs was similar to that of PCTNs, regardless of echogenicity or the presence of suspicious US features. Therefore, MCTNs could be categorized as PCTNs rather than as solid nodules to increase the accuracy of the risk stratification system for thyroid nodules.
NotesAuthor Contributions Conceptualization: Lee YJ, Kim JY, Na DG, Kim JH. Data acquisition: Lee YJ, Yoon RG, Kim SK, Bak S. Data analysis or interpretation: Lee YJ, Kim JY, Na DG, Kim JH, Oh M, Kim DB, Yoon RG, Kim SK, Bak S. Drafting of the manuscript: Lee YJ, Kim JY. Critical revision of the manuscript: Lee YJ, Na DG, Kim JH, Oh M, Kim DB, Yoon RG, Kim SK, Bak S. Approval of the final version of the manuscript: all authors. AcknowledgementsWe would like to express our gratitude to all doctors from 26 different hospitals who provided ultrasound data on thyroid nodules for the Thyroid Imaging Network of Korea registry
Ji Eun Shin1, Hye Shin Ahn2, Min Ji Hong2, Nami Choi3, Hwa Seon Shin4, Donghyun Kim5, Beomsu Kim6, Boeun Lee7, Hyun Jeong Kim8, Eun Kyoung Lee9, Wooyul Paik10, Jung Hyo Rhim11, A jung Chu11, Jong Yoon Lee11, Sun-Won Park11, Chang Yoon Lee12, Hyun Kyung Lim13, Jin Yong Sung14, Yeo Koon Kim15, Se Jin Cho15, Joon Hyung Lee16, So Lyung Jung17, Tae Yoon Kim18, Eun Ju Ha19, Yun Young Lee20, Jung Hwan Baek21, Young Jun Choi21, Sae Rom Chung21, Chong Hyun Suh21, Ji Ye Lee22, Inpyeong Hwang22, Roh-Eul Yoo22, Koung Mi Kang22, Tae Jin Yun22, Sung-Hye You23
1Department of Radiology, CHA University Gangnam Medical Center, 2Department of Radiology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, 3Department of Radiology, Konkuk University Medical Center, Konkuk University School of Medicine, 4Department of Radiology, Gyeongsang National University Hospital, 5Department of Radiology, Busan Paik Hospital, Inje University College of Medicine, 6Department of Radiology, Kosin University College of Medicine, 7Department of Radiology, Ewha Womans University Seoul Hospital, 8Department of Radiology, Daejeon St. MaryŌĆÖs Hospital, College of Medicine, The Catholic University of Korea, 9Department of Radiology, Dongguk University Ilsan Hospital, 10Department of Radiology, GangNeung Asan Hospital, University of Ulsan College of Medicine, 11Department of Radiology, Seoul Metropolitan Government, Seoul National University Boramae Medical Center, 12Department of Radiology, Research Institute and Hospital, National Cancer Center, 13Department of Radiology, Soonchunhyang University Seoul Hospital, 14Department of Radiology, Thyroid Center, Daerim Saint Mary's Hospital, 15Department of Radiology, Seoul National University Bundang Hospital, 16Department of Radiology, Inje University Haeundae Paik Hospital, 17Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, 18Department of Radiology, Hanyang University Guri Hospital, Hanyang University College of Medicine, 19Department of Radiology, Ajou University School of Medicine, 20Department of Radiology, Chonnam National University Hwasun Hospital, 21Department of Radiology, Asan Medical Center,University of Ulsan College of Medicine, 22Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, 23Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine
References1. Lee JY, Baek JH, Ha EJ, Sung JY, Shin JH, Kim JH, et al. 2020 Imaging guidelines for thyroid nodules and differentiated thyroid cancer: Korean Society of Thyroid Radiology. Korean J Radiol 2021;22:840ŌĆō860.
2. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81 Suppl 1:1ŌĆō122.
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1ŌĆō133.
4. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract 2016;22:622ŌĆō639.
5. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean Thyroid Imaging Reporting and Data System and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2021;22:2094ŌĆō2123.
6. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): a user's guide. Radiology 2018;287:29ŌĆō36.
7. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J 2017;6:225ŌĆō237.
8. Ha EJ, Na DG, Baek JH. Korean Thyroid Imaging Reporting and Data System: current status, challenges, and future perspectives. Korean J Radiol 2021;22:1569ŌĆō1578.
9. Na DG, Kim JH, Kim DS, Kim SJ. Thyroid nodules with minimal cystic changes have a low risk of malignancy. Ultrasonography 2016;35:153ŌĆō158.
10. Chung SR, Ahn HS, Choi YJ, Lee JY, Yoo RE, Lee YJ, et al. Diagnostic performance of the modified Korean Thyroid Imaging Reporting and Data System for thyroid malignancy: a multicenter validation study. Korean J Radiol 2021;22:1579ŌĆō1586.
11. Seo H, Na DG, Kim JH, Kim KW, Yoon JW. Ultrasound-based risk stratification for malignancy in thyroid nodules: a four-tier categorization system. Eur Radiol 2015;25:2153ŌĆō2162.
12. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892ŌĆō899.
13. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid Imaging Reporting and Data System risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid 2016;26:562ŌĆō572.
14. Salmaslioglu A, Erbil Y, Dural C, Issever H, Kapran Y, Ozarmagan S, et al. Predictive value of sonographic features in preoperative evaluation of malignant thyroid nodules in a multinodular goiter. World J Surg 2008;32:1948ŌĆō1954.
15. Lee MJ, Kim EK, Kwak JY, Kim MJ. Partially cystic thyroid nodules on ultrasound: probability of malignancy and sonographic differentiation. Thyroid 2009;19:341ŌĆō346.
An isoechoic and minimally cystic thyroid nodule without suspicious ultrasonography features in a 65-year-old woman.Transverse (A) and longitudinal (B) gray-scale sonograms show a minimally cystic thyroid nodule (cystic portion Ōēż10% of the entire nodule) with isoechogenicity, smooth margin, and parallel orientation in the left lobe (arrows, 32├Ś24├Ś17 mm). The final diagnosis confirmed a benign follicular nodule.
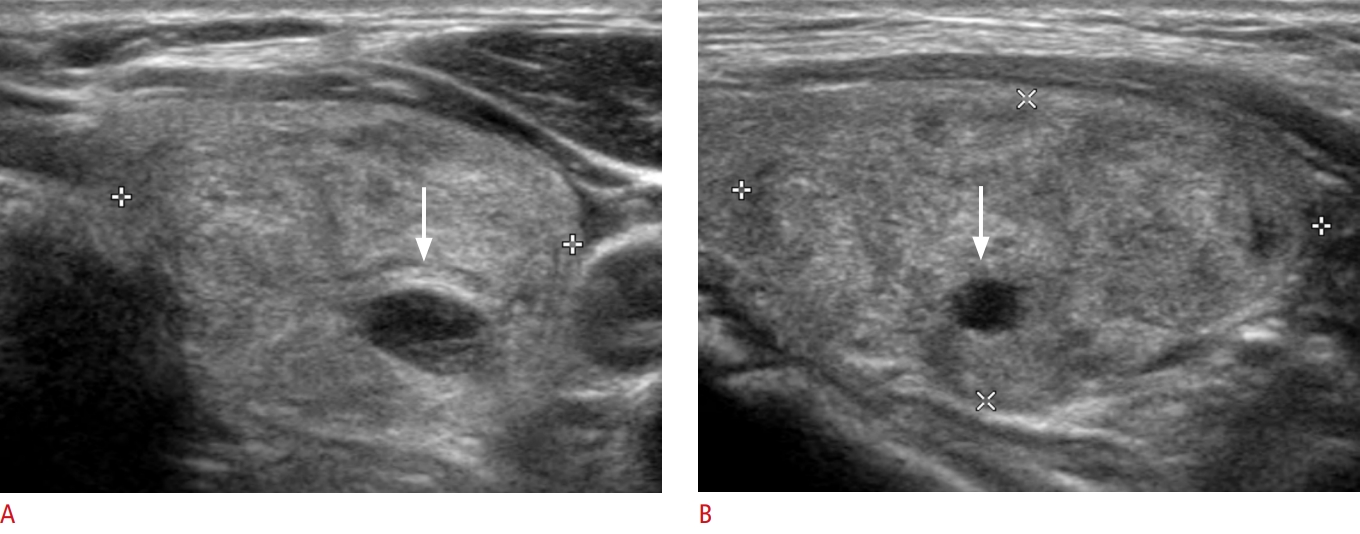 Fig.┬Ā2.A hypoechoic and minimally cystic thyroid nodule with suspicious ultrasonography features in a 70-year-old woman.Transverse (A) and longitudinal (B) gray-scale sonograms show a minimally cystic thyroid nodule (cystic portion Ōēż10% of the entire nodule) with mild hypoechogenicity, irregular margin, and nonparallel orientation (in the transverse plane) in the right lobe (arrows, 11├Ś12├Ś13 mm). The final diagnosis confirmed a papillary thyroid carcinoma.
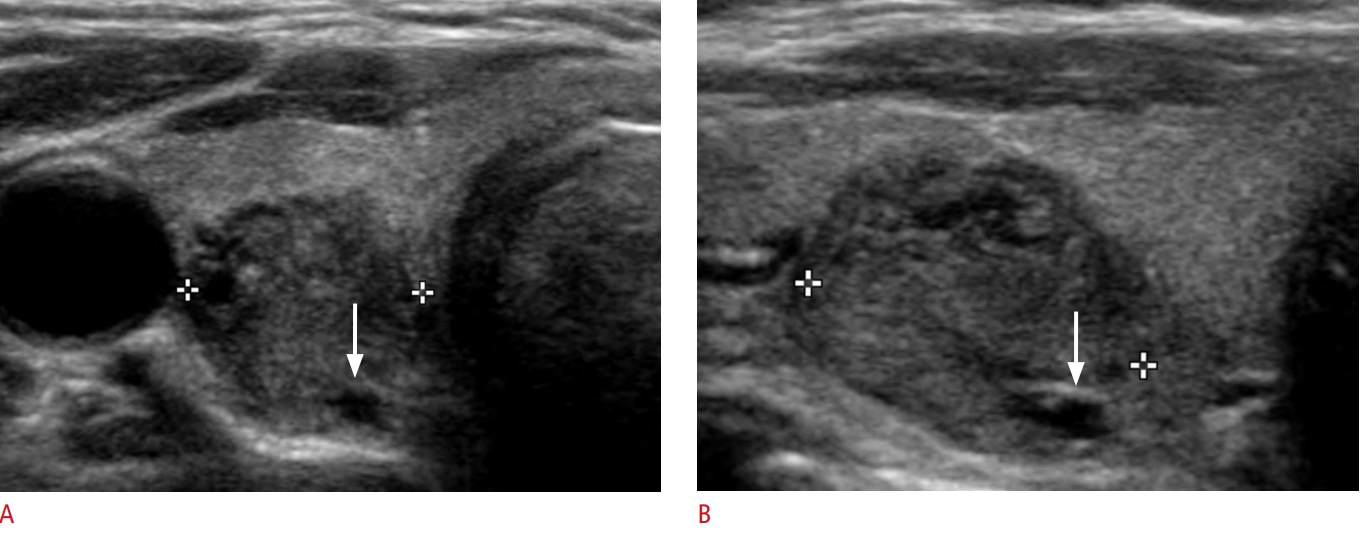 Fig.┬Ā3.Table┬Ā1.Demographic characteristics of 4,989 patients with 5,601 nodules Table┬Ā2.The frequency and malignancy risk of thyroid nodules according to composition Table┬Ā3.The frequency and malignancy risk of thyroid nodules according to composition and echogenicity Values are presented as number (%) unless otherwise indicated. The P-values comparing the malignancy risks in the hypoechoic nodule group: MCTNs vs. PCTNs (P=0.203) and MCTNs vs. purely solid nodules (P<0.001). The P-values comparing the malignancy risks in the isohyperechoic nodule group: MCTNs vs. PCTNs (P=0.092) and MCTNs vs. purely solid nodules (P<0.001). MCTN, minimally cystic thyroid nodule; PCTN, partially cystic thyroid nodule. Table┬Ā4.The frequency and malignancy risk of thyroid nodules according to composition and the presence of suspicious US features
Values are presented as number (%) unless otherwise indicated. US, ultrasonography; MCTN, minimally cystic thyroid nodule; PCTN, partially cystic thyroid nodule. a) Suspicious US features are punctated echogenic foci (microcalcification), nonparallel orientation (taller-than-wide shape), and irregular (spiculated/microlobulated) margin. The P-values comparing the malignancy risks in the group of nodules with suspicious US features: MCTNs vs. PCTNs (P=0.272) and MCTNs vs. purely solid nodules (P<0.001). The P-values comparing the malignancy risks in the group of nodules without suspicious US features: MCTNs vs. PCTNs (P=0.118) and MCTNs vs. purely solid nodules (P<0.001). Table┬Ā5.The frequency and risk of malignancy of thyroid nodules according to composition and nodule size
Values are presented as number (%) unless otherwise indicated. MCTN, minimally cystic thyroid nodule; PCTN, partially cystic thyroid nodule. a) P-values for comparing the malignancy risk between the large nodule group (>2 cm) and the small nodule group (Ōēż2 cm). The P-values comparing the malignancy risks in the small nodule group (Ōēż2 cm): MCTNs vs. PCTNs (P=0.093) and MCTNs vs. purely solid nodules (P<0.001). The P-values comparing the malignancy risks) in the large nodule group (>2 cm): MCTNs vs. PCTNs (P=0.056) and MCTNs vs. purely solid nodules (P<0.001). |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




