Groin abnormalities: ultrasonographic and clinical findings
Article information
Abstract
Groin lesions can be classified as neoplastic or non-neoplastic. Neoplastic lesions include lipoma, epidermoid cyst, angiomyofibroblastoma-like tumor, liposarcoma, and synovial sarcoma, as well as metastases from lymphoma, neuroendocrine carcinoma, and carcinomas of the lung, breast, urinary bladder, ovary, vulva, and colon. Non-neoplastic lesions include hernias, round ligament varices, endometriosis, Kimura disease, Castleman disease, hematoma, and inflammation. Because the clinical implications and therapeutic strategies for groin lesions vary depending on the cause, the ability to noninvasively differentiate among etiologies is very important. Although there is substantial overlap in ultrasonographic findings across various groin lesions, some ultrasonographic features, along with clinical characteristics, may suggest a specific diagnosis. Familiarity with the ultrasonographic and clinical features of various groin lesions facilitates accurate diagnosis and treatment.
Introduction
There are many diverse abnormalities of the groin, and the differential diagnosis of groin lesions can be puzzling for many clinicians because of their similar clinical presentations [1,2].
Ultrasonography is considered to be the first-line imaging modality for groin lesions. Ultrasonography is accurate for distinguishing between solid and cystic lesions and may play an important role in distinguishing between benign and malignant lesions.
In this article, we review the relevant literature and discuss the ultrasonographic and clinical features of groin lesions (Table 1). Groin lesions can be classified as either neoplastic or nonneoplastic. Neoplastic lesions include lipoma, epidermoid cyst, angiomyofibroblastoma-like tumor, liposarcoma, synovial sarcoma, and lymphoma, as well as metastases from neuroendocrine carcinoma and carcinomas of the lung, breast, urinary bladder, ovary, vulva, and colon. Non-neoplastic lesions include hernias, round ligament varices, endometriosis, Kimura disease, Castleman disease, hematoma, and inflammation.
Non-neoplastic Lesions
Hernia
Inguinal hernias are classified as direct or indirect depending on their relationship to the inferior epigastric artery. Indirect inguinal hernias originate at the deep inguinal ring, lateral to the inferior epigastric vessels, and follow the course of the inguinal canal. Direct inguinal hernias pass through a defect of the Hasselbach triangle, which is medial to the inferior epigastric artery. Hernia contents include bowel loops, omental fat, and ovarian and peritoneal fluid [1,2]. On ultrasonography, hernia contents can be hyperechoic due to omental fat (Fig. 1), anechoic due to fluid, or have mixed echogenicity due to reverberations of air in the bowel loops [1,2].

A 3-year-old girl with a left inguinal hernia containing omental fat.
Transverse ultrasonography shows an ovoid-shaped hyperechoic lesion in the left inguinal region (arrows).
Pseudomyxoma peritonei occurs as result of a ruptured appendiceal mucocele or intraperitoneal spread of mucinous tumors, including mucinous adenocarcinomas of the ovary, appendix, and colon [3]. In patients with pseudomyxoma peritonei, the tumor may metastasize transcoelomically to implant within the peritoneal lining of the hernia sac [3]. If the hernia contents are echogenic with septated ascites and an irregularly thickened peritoneum on ultrasonography, there is a high possibility of pseudomyxoma peritonei (Fig. 2) [3].

A 91-year-old man with pseudomyxoma peritonei caused by mucinous cystadenocarcinoma of the appendix.
A. Transverse ultrasonography shows a cystic lesion with internal echogenic foci and thickened wall in the right groin. B, C. Transverse and coronal contrast-enhanced computed tomography show a tubular cystic mass in the right groin (arrow). D. Photomicrography shows an abundant mucin pool with a lining of atypical mucinous glandular cells. The atypical mucinous glandular epithelium shows infiltration of stroma (H&E, ×100).
Spermatic Cord Hydrocele
Spermatic cord hydrocele is a loculated fluid collection along the spermatic cord that results from failure of closure of the processus vaginalis [4]. Two types of cord hydrocele exist: encystic hydrocele and funicular hydrocele. On ultrasonography, encystic hydrocele is a loculated fluid collection within the inguinal canal that does not communicate with the peritoneal cavity (Fig. 3). Funicular hydroceles do communicate with the peritoneal cavity (Fig. 4) [4].

A 2-year-old boy with an encystic hydrocele.
Longitudinal ultrasonography shows an ovoid-shaped, cystic lesion in the left spermatic cord.
Round Ligament Varices
The round ligament extends from the lateral uterus to the labia majora and contains arteries, veins, lymphatic vessels, and nerves. Round ligament varices are prominent veins within the round ligament and are more common in pregnancy because of increased venous flow and reduced venous tone [5]. In addition, progesterone receptors are normally present within the round ligament veins, and pregnancy-induced increases in progesterone levels cause dilatation of these veins [5].
Ultrasonography reveals multiple cystic lesions with a "bag of worms" appearance associated with dilated veins (Fig. 5A) [5]. Color Doppler ultrasonography can confirm the venous flow (Fig. 5B) [5,6]. Differentiating these varices from inguinal hernias is important because the management of these two conditions is different [6].
Endometriosis
Endometriosis is characterized by the proliferation of endometrial tissue at ectopic sites. If the canal of Nuck, a small evagination of the parietal peritoneum that accompanies the round ligament through the inguinal ring into the inguinal canal, is not obliterated, the endometrial tissue may implant in the inguinal area through the canal of Nuck [7]. Most patients have painful swelling of the inguinal region [7].
The ultrasonographic appearance of inguinal endometriosis is variable, including solid masses (Fig. 6), cystic masses (Fig. 7), and combined cystic and solid masses [7].

A 29-year-old woman with endometriosis.
Transverse ultrasonography shows a hypoechoic mass with irregular margins in the right groin.
Kimura Disease
Kimura disease is an idiopathic chronic inflammatory disorder that usually involves the head and neck area, such as the salivary glands and oral cavity. However, it may also occur in the axilla, groin, trunk, abdomen, and peripheral limbs [8]. The clinical features of Kimura disease are painless subcutaneous soft tissue masses, peripheral eosinophilia, and elevated serum immunoglobulin E levels [8].
On ultrasonography, it presents as a homogeneously hypoechoic round mass with normal hilar architecture (Fig. 8) [8]. On color Doppler ultrasonography, the disease usually manifests as profuse hilar vascularity with low resistance [9].

A 49-year-old woman with Kimura disease.
A. Transverse ultrasonography shows a hypoechoic mass with irregular margins in the right groin. B. Contrast-enhanced computed tomography shows a soft tissue mass in the right groin. C. A photomicrograph shows marked infiltration of eosinophils and lymphocytes with a germinal center (H&E, ×100).
Castleman Disease
Castleman disease is a rare lymphoproliferative disorder and is alternatively known as angiofollicular lymph node hyperplasia or giant lymph node hyperplasia [10]. Castleman disease is classified as unicentric or multicentric based on the extent of lymph node involvement [10]. It can be classified as hyaline vascular, plasma cell, human herpes virus 8-associated, or multicentric Castleman disease not otherwise specified [10], and occurs in the chest (70%), neck (15%), or abdomen and pelvic regions (15%) [10].
Castleman disease is usually seen as a well-defined, homogeneously hypoechoic mass on ultrasonography (Fig. 9A) [11]. Color Doppler ultrasonography reveals a hypervascular mass (Fig. 9B) [11].

A 55-year-old man with Castleman disease.
A, B. Transverse gray-scale and color Doppler ultrasonography show a heterogeneously hypoechoic mass (arrows) with increased internal blood flow in the right groin. C. Contrast-enhanced computed tomography shows an enhancing soft tissue mass in the right groin (arrow).
Hematoma and Inflammation
The echogenicity of hematoma varies according to stage. Initially, the hematoma is hyperechoic, but then becomes gradually hypoechoic and anechoic (Fig. 10) [2].

A 65-year-old man with a hematoma after laparoscopic herniorrhaphy.
Transverse ultrasonography shows a mixed anechoic and hyperechoic mass in the right groin.
Inflammation and abscesses may occur in the groin. Ultrasonography shows variable echogenicity, which ranges from anechoic to hypoechoic (Fig. 11). The differentiation of tumors, hematomas, and inflammation may be difficult on ultrasonography.
Neoplastic Lesions
Benign Tumors
Lipoma is the most common benign tumor of the groin. On ultrasonography, it can have variable echogenicity compared with the surrounding soft tissues, ranging from hyperechoic to hypoechoic (Fig. 12). It may be difficult or impossible to differentiate from liposarcoma [1,2].

A 61-year-old man with lipoma.
Longitudinal ultrasonography shows a mixed hyperechoic and hypoechoic mass in the left groin (arrows).
Epidermoid cyst is a rare congenital lesion of ectodermal origin that usually affects middle-aged women and can occur anywhere from the head to toe. It is known to have a component of stratified squamous epithelium with a mixture of desquamated debris, cholesterol, keratin, and water. Ultrasonography shows a heterogeneously cystic mass containing echogenic debris (Fig. 13) [12]. The echogenicity varies according to the composition of the lesion [13].

A 42-year-old man with an epidermoid cyst.
Longitudinal ultrasonography shows an ovoid-shaped, hypoechoic mass with internal echoes and posterior enhancement in the right groin.
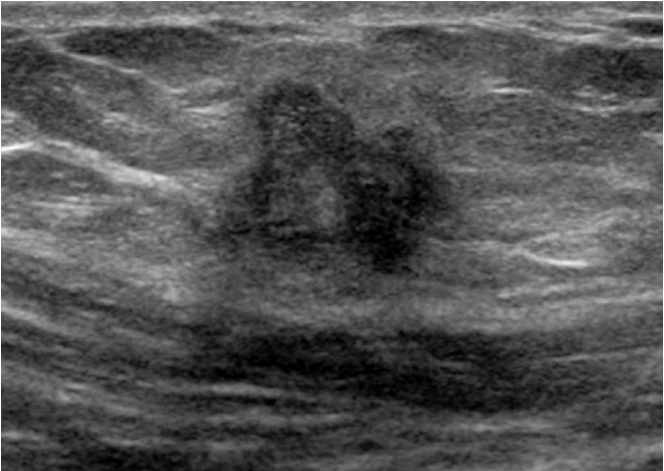
Angiomyofibroblastoma-like tumor or cellular angiofibroma is a rare mesenchymal tumor that occurs in the inguinal area, perineum, scrotum, and vulva [14]. It has been suggested that its histogenesis arises from perivascular stem cells with a capacity for fatty and myofibroblastic differentiation [14]. This disease commonly occurs during the fifth to eighth decades of life [14]. On ultrasonography, the mass usually presents as a well-defined mass in the subcutaneous layer of the inguinal region that is heterogeneous and isoechoic to the surrounding subcutaneous fat [15]. It is usually hypervascular on color Doppler ultrasonography [15]. In a case that we experienced, the mass appeared hypoechoic and hypervascular (Fig. 14). Other benign tumors of the groin include leiomyoma, neurofibroma, desmoid tumor, dermoid cyst, and lymphangioma.

A 43-year-old man with an angiomyofibroblastoma-like tumor.
A, B. Longitudinal gray-scale and color Doppler ultrasonography show an ovoid-shaped hypoechoic lesion with internal blood flow in the left inguinal region. C. A microphotograph shows bland spindle cells with scant, lightly eosinophilic cytoplasm with ill-defined borders. Additionally, prominent small to medium-sized vessels with fibrous stroma are seen (H&E, ×100).
Malignant Tumors
Most malignant tumors in the groin are sarcomas and the most common sarcomas are rhabdomyosarcoma and liposarcoma [16]. The former frequently occurs in children and the latter is more common in adults [16].
Liposarcoma is a bulky yellow tumor similar to lipoma but is generally more complex and contains areas of prominent sclerosis [16]. Liposarcoma can be classified as well differentiated, dedifferentiated, myxoid, pleomorphic, or not otherwise specified. The ultrasonographic findings of liposarcoma are variable and nonspecific (Fig. 15) [16].

A 45-year-old man with myxoid liposarcoma.
Longitudinal ultrasonography shows a well-defined hypoechoic mass with tiny cystic foci (arrows) in the left groin.
Synovial sarcoma is a mesenchymal tumor of uncertain pathogenesis that accounts for 10% of all primary soft tissue malignant tumors. Although the tumor often occurs close to joints, tendons, and bursae, it can appear in a variety of locations, including the head and neck, retroperitoneum, mediastinum, and groin [17]. Synovial sarcoma can occur at all ages but is most commonly seen in young adults and adolescents. It usually appears as a solid mass (Fig. 16) [2], although it may also appear as a cystic mass [18].

A 45-year-old woman with synovial sarcoma.
A, B. Transverse gray-scale and color Doppler ultrasonography show a well-defined, hypoechoic mass with increased internal blood flow in the left groin. C. A microphotograph shows atypical spindle cell nests surrounded by a thin fibrous capsule. The tumor cells have pleomorphism and prominent nucleoli (H&E, ×200).
Other primary malignant tumors involving the groin include malignant fibrous histiocytoma, fibrosarcoma, and lymphoma (Fig. 17) [19].

A 76-year-old woman with non-Hodgkin lymphoma.
A, B. Transverse gray-scale and color Doppler ultrasonography show a hypoechoic mass with increased blood flow within the mass in the left groin. C. Contrast-enhanced computed tomography shows a soft tissue mass in the left groin (arrow).
Secondary metastatic disease from melanoma, neuroendocrine carcinoma (Fig. 18), and carcinomas of the lung, breast, urinary bladder (Fig. 19), ovary, vulva, and colon can occur in the groin [1,2]. The ultrasonographic findings of these masses are usually hypoechoic [2]. Although lymphoma is markedly hypoechoic [20], the differential diagnosis of these masses is difficult. Metastatic masses should be distinguished from benign lymph nodes. On ultrasonography, a normal lymph node usually appears as an oval mass with a hypoechoic peripheral zone and echogenic center [20]. Metastatic lymph nodes usually have a round shape, normal or absent hilus, and eccentric widening of the cortex on gray-scale ultrasonography, and a high resistive index and pulsatility index on color Doppler ultrasonography [20].

A 68-year-old man with metastatic neuroendocrine carcinoma.
A, B. Transverse gray-scale and color Doppler ultrasonography show a well-defined, round, heterogeneously hypoechoic mass with increased internal blood flow in the right groin. C. A microphotograph shows atypical cell nests with uniform tumor cells. The tumor cells have many mitotic figures and prominent nucleoli (H&E, ×200).
Conclusion
In patients with groin lesions, ultrasonography is considered to be the first-line imaging modality and may provide important information about the anatomical location, size, and whether the lesion is solid or cystic. However, there is substantial overlap in the ultrasonographic findings of various groin lesions. Although ultrasonography is nonspecific for distinguishing between many kinds of groin lesions, familiarity with the ultrasonographic findings of groin lesions facilitates accurate diagnosis and treatment.
Notes
Author Contributions
Conceptualization: Yang DM. Data acquisition: Yang DM, Kim HC, Kim SW. Data analysis or interpretation: Yang DM, Kim HC, Kim SW, Won KY. Drafting of the manuscript: Yang DM, Won KY. Critical revision of the manuscript: Yang DM, Kim HC, Kim SW, Won KY. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.