Clinical applications of Doppler ultrasonography for thyroid disease: consensus statement by the Korean Society of Thyroid Radiology
Article information
Abstract
Doppler ultrasonography (US) is widely used for the differential diagnosis of thyroid nodules, metastatic cervical lymph nodes in patients with thyroid cancer, and diffuse parenchymal disease, as well as for guidance in various US-guided procedures, including biopsy and ablation. However, controversies remain regarding the appropriate use and interpretation of Doppler US. Therefore, the Korean Society of Thyroid Radiology organized a taskforce to develop a consensus statement on the clinical use of Doppler US for thyroid disease. The review and recommendations in this article are based on a comprehensive analysis of the current literature and the consensus of experts.
Introduction
Doppler ultrasound (US) is an imaging technique that exploits the shift in the frequency of US waves when they are reflected by moving blood (the Doppler effect) [1]. Doppler US can provide information on perithyroidal vessels and the vascularity of thyroid lesions and is widely used in daily practice for the evaluation of thyroid disease alongside gray-scale US. There are several types of Doppler US techniques, including spectral Doppler US (SDUS), color Doppler US (CDUS), power Doppler US (PDUS), and superb microvascular imaging (SMI). Many studies have used these techniques and reported different results regarding focal thyroid nodules and diffuse parenchymal disease [2-11]. Doppler US is also widely used for US-guided procedures, such as biopsy and ablation of thyroid nodules [3-7,12,13]. With the increasing use of these procedures, there is a need for a consensus statement on the clinical applications of Doppler US for thyroid disease.
In March 2019, the Korean Society of Thyroid Radiology organized a taskforce to develop a consensus statement on the clinical applications of Doppler US for thyroid disease. The recommendations include the basic principles and techniques of Doppler US and their clinical applications for thyroid nodules, metastatic cervical lymph nodes in patients with thyroid cancer, diffuse parenchymal disease, and US-guided procedures. A PubMed/MEDLINE search using the keywords "thyroid," "Doppler," "vascular," and "ultrasound" was performed to retrieve publications from January 2000 to April 2019. The draft consensus statement for the clinical application of Doppler US for thyroid disease was posted on the official website (https://www.thyroidimaging.kr/) and was reviewed by members of the Korean Society of Thyroid Radiology. The goal of these recommendations is to summarize the best scientific evidence available and to provide a consensus expert opinion on the use of Doppler US for thyroid disease.
Basic Principles and Techniques of Doppler US
Doppler US is based on the Doppler effect and utilizes the change in the frequency of a sound wave resulting from motion. In Doppler US, the Doppler effect allows one to detect the movement of red blood cells in the blood vessels. Thus, Doppler US provides information on blood flow, which is related to the vascularization of anatomical structures. Several Doppler US techniques have been used to evaluate thyroid gland lesions, including SDUS, CDUS, PDUS, and SMI.
SDUS displays a spectrum of flow velocities represented graphically on the Y-axis against time on the X-axis. Thus, the spectral mode allows us to measure different blood flow parameters. CDUS involves a combination of the Doppler effect and real-time US imaging. In CDUS, the information from the Doppler technique is integrated with the B-mode (i.e., gray-scale) image to form a color signal with the colors indicating the direction of flow; red usually indicates flow toward the transducer and blue indicates flow away from the transducer. PDUS displays the power of the Doppler shift in small volumes within the field of view, while CDUS displays the mean Doppler shift. Therefore, PDUS potentially offers increased sensitivity for detecting low-velocity flow in small vessels [14]. SMI deals with the issue that Doppler signals are derived not only from the blood flow, but also from tissue motion (clutter); as a result, the clutter signals overlap the low-speed flow components. Conventional Doppler techniques apply a single-dimensional wall filter to remove clutter, resulting in loss of the slow component. In contrast, SMI uses a multidimensional filter to separate flow signals from clutter, thus removing only the clutter while preserving the slow-flow signals [12]. Additionally, contrast-enhanced US (CEUS) refers to the application of US contrast agents in medical US. CEUS can provide anatomic information with gray-scale US and physiologic information through the SDUS, CDUS, and PDUS techniques, making it indispensable in the assessment of vascular pathology [15-21]. The presence of a microbubble US contrast agent in the vasculature enhances the backscattering of US waves, resulting in a marked amplification of flow signals and providing information on the microvasculature [12,21,22].
Clinical Applications of Doppler US
Diagnosis of Focal Thyroid Nodules
Differential diagnosis of benign and malignant thyroid nodules
Color Doppler US
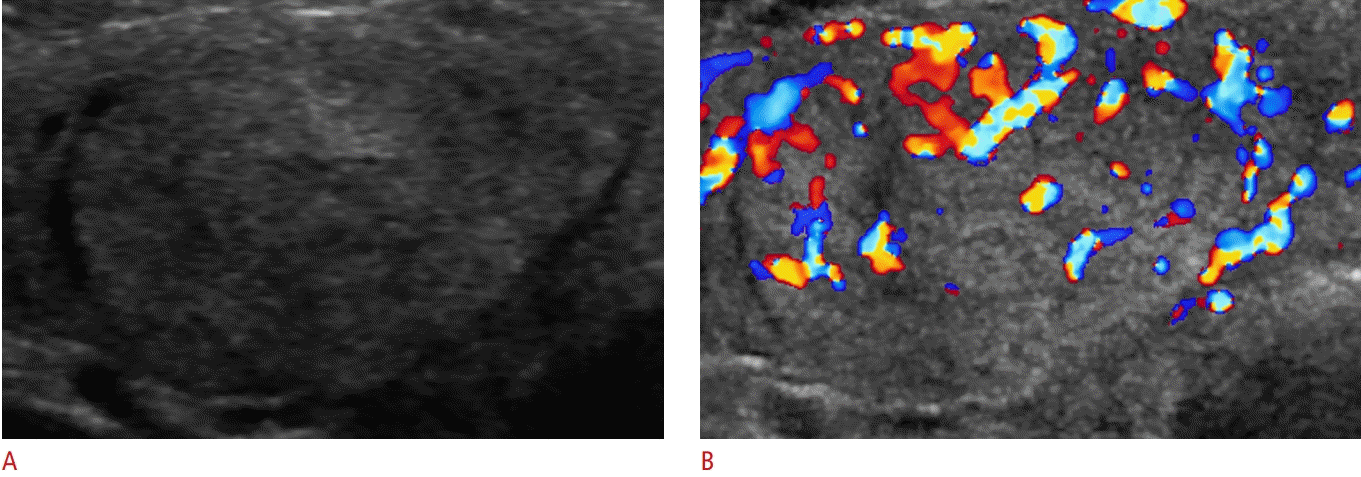
The vascularity patterns of thyroid nodules on CDUS can be categorized into four types: type 1, absence of nodule vascularity; type 2, perinodular vascularity only (presence of circumferential vascularity at the margin of a nodule); type 3, mild intranodular vascularity with or without perinodular vascularity (vascularity <50%); and type 4, marked intranodular vascularity with or without perinodular vascularity (vascularity >50%) (Fig. 1) [8].
Vascular patterns of thyroid nodules on color Doppler ultrasonography.
The patterns are categorized into four types: type 1, absence of thyroid nodule vascularity (A); type 2, perinodular vascularity only (presence of circumferential vascularity at the margin of a thyroid nodule) (B); type 3, mild intranodular vascularity with or without perinodular vascularity (vascularity <50%) (C); type 4, marked intranodular vascularity with or without perinodular vascularity (vascularity >50%) (D).
CDUS can be clinically applied for the differential diagnosis of benign and malignant thyroid nodules, as it has high sensitivity and specificity [23-28]. Papini et al. [24] prospectively evaluated the predictive value of CDUS in 494 patients and reported that intranodular vascular spots (odds ratio, 14.23) were an independent risk factor of malignancy. In another prospective study including 90 patients, Ebeed et al. [29] reported similar results, observing CDUS patterns of obvious intranodular blood flow with absent or slight perinodular blood flow (type 3) in malignant thyroid nodules, with sensitivity, specificity, and accuracy for malignancy of 79.2%, 95.6%, and 88.7%, respectively. In two retrospective studies, extensive internal flow was associated with malignant thyroid nodules [25,30]; one study reported that the sensitivity, specificity, and accuracy of CDUS for the detection of malignant thyroid nodules were 93.6%, 86.7%, and 90.7%, respectively [25]. However, in a prospective study by Hong et al. [27] that included 243 patients, marked intranodular vascularity, defined as greater flow in the central part of the nodule than in the periphery, had a disappointingly low diagnostic performance (sensitivity of 31% and specificity of 81%). They also demonstrated that marked intranodular vascularity was more commonly observed in large thyroid nodules than in small malignant or benign thyroid nodules (P=0.01 for malignant nodules and P=0.001 for benign nodules) [27]. In a recently published meta-analysis of 20 studies, CDUS showed a pooled sensitivity of 74% (95% confidence interval [CI], 62%-83%) and a pooled specificity of 70% (95% CI, 56%-81%) for the diagnosis of malignant nodules and an area under the curve (AUC) of 0.78 (95% CI, 0.74-0.81) on summary receiver operating characteristic (ROC) curve analysis [23]. In summary, CDUS shows acceptable diagnostic performance and may be useful for predicting malignant thyroid nodules. Marked intranodular vascularity on CDUS is a significant predictor of malignancy in thyroid nodules [23-30].
Power Doppler US
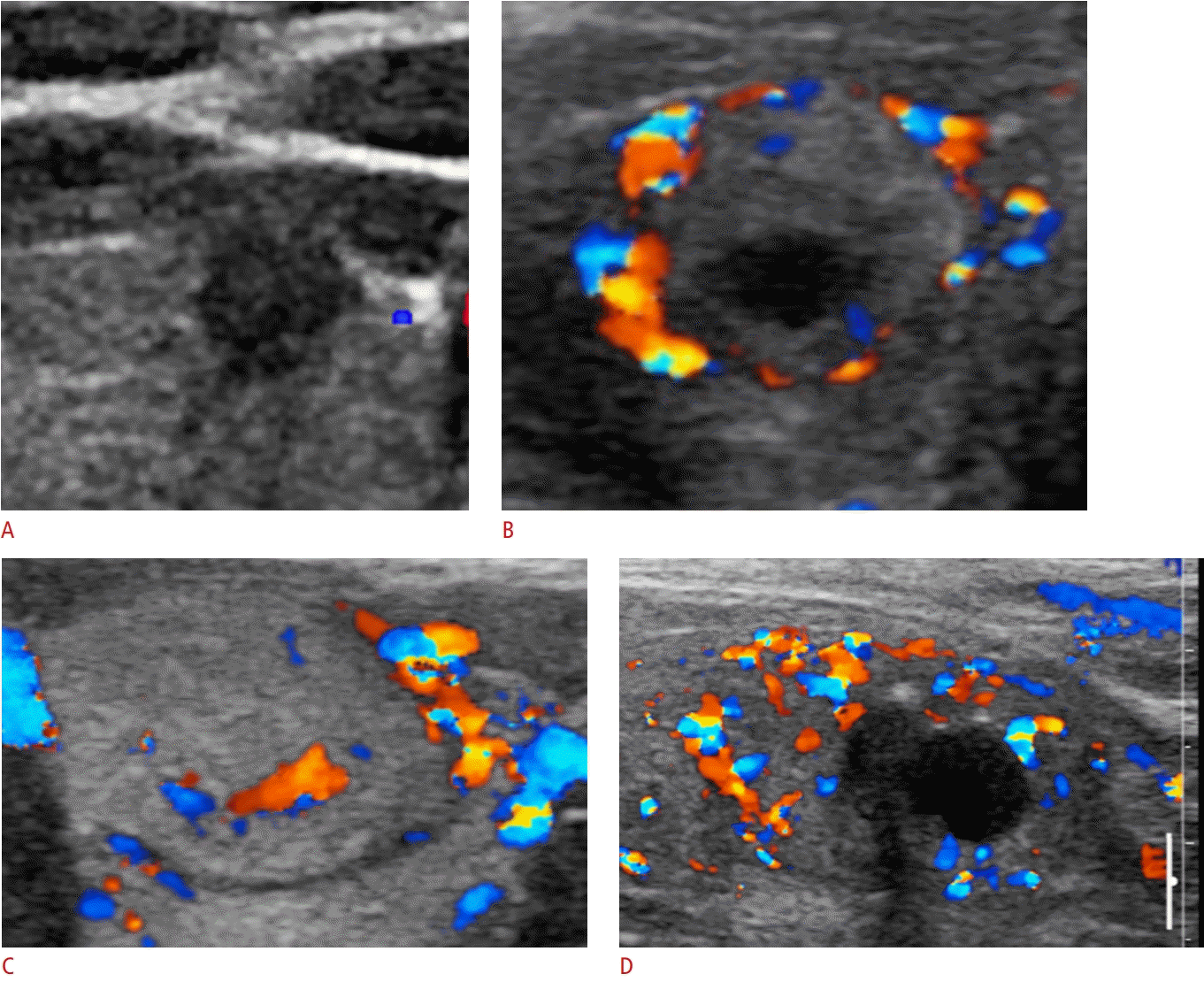
PDUS performed with a high-frequency transducer allows identification of low-velocity blood flow in superficial organs, such as the thyroid gland [31-34]. PDUS may be more sensitive than CDUS in detecting the slow flow of small vessels (Fig. 2) [31,34]. Several previous studies have suggested that PDUS is highly sensitive and specific in differentiating between benign and malignant thyroid nodules [31-33]. Chammas et al. [31] evaluated the predictive value of PDUS in 177 thyroid nodules from 174 patients and reported that nodules with central or mainly central vascularization patterns (type 4 or 5) were an independent risk factor for malignancy (odds ratio, 219). Several previous studies have demonstrated that the risk of thyroid malignancy increases as marked intranodular blood flow becomes more predominant [31-33]. However, these results are inconsistent with those of other studies that showed an absence of blood flow signal in papillary carcinomas [35-37]. A retrospective study by Moon et al. [37] including 1,083 thyroid nodules evaluated three patterns of vascularity (none, peripheral, and intranodular) on PDUS. The authors reported that intranodular vascularity was frequently seen in benign thyroid nodules, while an absence of vascularity was more frequent in malignant thyroid nodules (P<0.001) and that vascularity itself or a combination of vascularity and gray-scale US features was not as useful as suspicious gray-scale US features alone for predicting thyroid malignancy [37]. The use of the flow pattern on PDUS to predict thyroid malignancies is controversial.
Spectral Doppler US
SDUS displays a spectrum of flow velocities; therefore, it can provide information on multiple blood flow parameters. The peak systolic velocity (PSV), mean systolic velocity (MSV), and end diastolic velocity (EDV) are measured, while the resistive index (RI) and pulsatile index (PI) are calculated using the following formulas [28,38]:
Several studies have reported that when combined with gray-scale US, SDUS can improve the diagnosis of thyroid malignancy [28,38]. In a prospective study by Palaniappan et al. [28] evaluating the predictive value of SDUS in 214 thyroid nodules of 194 patients, the mean RI and PI of malignant thyroid nodules was 0.73 and 1.3, respectively, which were significantly higher than those of benign nodules, and the sensitivity, specificity, and accuracy of SDUS for the diagnosis of malignant thyroid nodules were 76.8%, 81.7%, and 80.6%, respectively. An additional cross-sectional study confirmed these results and reported a significant association between PI and malignancy, using a PI cutoff of ≥0.945 (P=0.007) for determining malignancy [38]. Chammas et al. [31] reported similar results-an RI>0.77 was an independent risk factor for malignancy of a nodule (odds ratio, 4.1) and a combination of PDUS and RI had a diagnostic sensitivity and specificity of 92.3% and 88%, respectively, for the detection of malignant thyroid nodules. Further, another study by Algin et al. [39] showed that the RI and PI values were higher in malignant thyroid nodules than in benign thyroid nodules (P<0.05) [39]. In summary, the RI and PI values of SDUS are significantly higher in malignant nodules than in benign nodules, and SDUS has acceptable diagnostic performance for the prediction of malignant thyroid nodules.
Contrast-enhanced US
CEUS is widely used to evaluate microvascularization and tumor angiogenesis, which are helpful for the differential diagnosis of malignancy and benignity [40,41]. CEUS was first used to evaluate focal liver lesions when B-mode and Doppler US yielded equivocal results [42]. With improvements in US equipment and the introduction of second-generation contrast agents (e.g., SonoVue [Bracco Imaging, Milan, Italy]) for the characterization of malignant thyroid nodules, the accuracy of CEUS for predicting thyroid malignancy has improved and its use has increased [43,44]. The following is the general process of CEUS for thyroid nodules. The focus zone is always placed at the bottom level of the nodule, and CEUS is performed using a low mechanical index (<0.10). The contrast agent is injected intravenously as a bolus, followed by a saline flush. The timer on the US machine is started during the CEUS process, and the images are obtained during the next 2-3 minutes [44].
The degree of enhancement of thyroid nodules on CEUS is divided into three patterns in reference to the surrounding parenchyma: hyperenhancement, isoenhancement, and hypoenhancement [44]. Hypoenhancement is a major CEUS pattern characteristic of malignant thyroid nodules [44-47]. When hypoenhancement was used as the diagnostic criterion for malignant thyroid nodules, the sensitivity, specificity, and accuracy of CEUS were 82.1%-89.8%, 80%-91.8%, and 84%-91%, respectively [45-47]. The main reason for hypoenhancement in malignant thyroid nodules is the lack of blood supply in a nodule because of necrosis and embolus formation within the tumor [44]. Another important CEUS pattern characteristic of malignant thyroid nodules is heterogeneous enhancement [44-46,48,49]. When heterogeneous enhancement was used as the diagnostic criterion for thyroid malignancy, the sensitivity, specificity, and accuracy of CEUS were 88%-90.4%, 80%-92.5%, and 85%-91%, respectively [45,46,48,49]. The blood vessels of malignant nodules are typically aberrant and tortuous, whereas those of benign nodules are usually regular [48]. Furthermore, most malignant thyroid nodules include areas of calcification, fibrosis, and/or necrosis, which are related to heterogeneous enhancement [44]. In addition to qualitative assessments of thyroid nodules, quantitative assessments are important for predicting malignancy with CEUS. CEUS with time-intensity curve analysis is helpful in diagnosing malignant thyroid nodules [44,50,51]. Chen et al. [51] evaluated the CEUS characteristics of 130 papillary thyroid microcarcinomas in 106 patients and reported that the peak enhancement intensity was lower than that of normal thyroid parenchyma and that contrast washed out faster from papillary thyroid microcarcinomas than from normal thyroid parenchyma. Furthermore, Nemec et al. [52] found that relative enhancement during the washout curve was lower in malignant thyroid nodules than in benign nodules, with an optimal cutoff value of 2.35 for predicting malignancy. Using this cutoff value, the diagnostic performance of CEUS had 76.9% sensitivity, 69.7% specificity, and 69.6% accuracy [52].
On CEUS, hypoenhancement and heterogeneous enhancement are major findings of malignant thyroid nodules [44-49]. CEUS with time-intensity curve analysis is also helpful in distinguishing benign from malignant thyroid nodules [50-52].
Superb microvascular imaging
SMI analyzes clutter motion and uses a new algorithm to identify and remove tissue motion, allowing the imaging of microvascular blood flow [53]. SMI depicts perinodular and intranodular thyroid microvascular flow in higher detail than conventional CDUS or PDUS [54,55]. Zhu et al. [55] prospectively compared SMI, gray-scale US, and CDUS in distinguishing between benignity and malignancy in 76 thyroid nodules and reported that SMI was significantly more accurate for identifying malignant thyroid nodules (79.3%) than CDUS (55.2%; P<0.001). Gray-scale US with SMI resulted in the greatest diagnostic sensitivity, specificity, and accuracy (86.2%, 85.1%, and 85.5%, respectively), and the AUC value for ROC analysis of gray-scale US with SMI (0.911 [95% CI, 0.849-0.973]) was significantly better than those of gray-scale US with CDUS or gray-scale US alone [55]. Vascularity on SMI is classified into three types: none, peripheral, and mixed and intranodular [56]. Kong et al. [56] evaluated the vascularity of 113 thyroid nodules and showed that for the diagnosis of thyroid malignancies, intranodular vascularity on SMI had a 91.2% specificity and 75.9% sensitivity, superior to the 82.3% specificity and 41.8% sensitivity of PDUS (P<0.01). The authors demonstrated that a taller-than-wide shape, microcalcifications, and intranodular vascularity on SMI were independent risk factors for thyroid malignancy [56]. An alternative classification of the vascularity on SMI used the number of vessels inside the thyroid nodule, with a score of 1 for a maximum of two blood vessels on SMI and a score of 2 for three or more vessels on SMI [54]. Zhu et al. [55] showed that a score of 2 was found in 40.4% benign nodules and 82.1% malignant nodules (sensitivity, 81.7%; specificity, 60.5%) and that an SMI score of 2 was an independent risk factor for thyroid malignancy (P<0.001). In summary, SMI is an emerging additional modality to conventional US. SMI is more accurate than CDUS or PDUS for the assessment of malignant thyroid nodules, and SMI findings of a large number of vessels and intranodular flow are associated with malignancy in thyroid nodules [54-56].
Diagnosis of follicular neoplasms
Follicular thyroid carcinoma accounts for 10%-20% of all thyroid malignancies. Most cases of follicular thyroid carcinoma require surgical biopsy or excision to determine the diagnosis [57]; however, >80% of all thyroid follicular neoplasms are benign [58]. CDUS is useful for predicting malignant thyroid follicular neoplasms [2,59]. The most hypervascular thyroid nodule types are adenoma/adenomatoid nodules, the encapsulated subtype of the follicular variant of papillary thyroid carcinoma, and follicular carcinoma [60]. A meta-analysis of CDUS for predicting malignant thyroid follicular neoplasms [2] showed an overall sensitivity of 85% and specificity of 86% and found that a predominant internal flow seen on CDUS was associated with malignant thyroid follicular neoplasms (Fig. 3). Fukunari et al. [60] used a CDUS and SDUS diagnostic grading system for the differential diagnosis of thyroid follicular lesions as follows: grade 1, benign follicular lesion (no color flow inside the nodule); grade 2, benign peripheral type (color flow only in the peripheral area, PI<1.0); grade 3, suspected follicular carcinoma (penetrating color flow, moderate vascularity); and grade 4, follicular carcinoma (high-velocity penetrating color flow, PI=1.0). On the assumption that grade 1 and 2 lesions are benign and grade 3 and 4 lesions are malignant, the sensitivity, specificity, and accuracy for predicting malignant thyroid follicular neoplasms were 88.9%, 74.2%, and 81.0%, respectively [2]. Additionally, Miyakawa et al. evaluated the vascular pattern of PDUS and the parameters of SDUS and reported that the majority (84%) of follicular adenomas showed only a peripheral rim of color flow on PDUS and that the PI and RI values on SDUS were significantly higher in patients with follicular carcinoma than in those with follicular adenoma (P<0.005 and P<0.001, respectively). Follicular carcinoma can be diagnosed with a cutoff values of PI >1.35 and RI >0.78, respectively, with AUC values of 0.898 for PI and 0.876 for RI on ROC analysis [61]. In summary, Doppler US may be a useful additional method for differentiating benign from malignant thyroid follicular neoplasms; predominant internal blood flow is seen in the latter [2,59,60]. SDUS parameters are also helpful for the diagnosis of malignant thyroid follicular neoplasm [60,61], with PI and RI being higher in malignant thyroid follicular neoplasms than in benign thyroid follicular neoplasms [60,61]. However, predominant internal blood flow can also be seen in benign thyroid nodules. Using predominant internal blood flow, the overall sensitivity for predicting thyroid malignancy was very high (96%), although the specificity was very low (14%), with positive and negative predictive values of 15% and 96%, respectively [2]. A systematic review and metaanalysis reported that CDUS had an overall sensitivity of 85% and a specificity of 86% for thyroid follicular neoplasms, with positive and negative predictive values of 51% and 97%, respectively [2].
Diagnosis of metastatic cervical lymph nodes in patients with thyroid cancer
Diagnosing metastatic lymph nodes in patients with thyroid cancer is important when planning surgery. Gray-scale US has been established as a primary imaging modality. Moreover, the combination of Doppler US and gray-scale US can increase the diagnostic accuracy. Hilar or central vascularity of the lymph node is a typical finding of a benign lymph node. In contrast, peripheral or diffuse vascularity is a US characteristic of lymph nodes with metastasis from thyroid cancer, and the absence of central hilar vascularity is a US criterion for an indeterminate lymph node [8,62]. Leboulleux et al. [63] prospectively evaluated the diagnostic accuracy of US for lymph node metastasis in 56 lymph nodes from 19 patients with differentiated thyroid cancer. They reported that the pattern of vascularity (peripheral vascularity in PDUS) had the best sensitivity and specificity (86% and 82%, respectively) among the US findings, making the use of Doppler US essential in the follow-up of differentiated thyroid cancer [63]. A previous study of CEUS showed similar results, in which heterogeneous, centripetal, and hybrid (mixture of centripetal and centrifugal) enhancement were significantly related to lymph node metastasis and CEUS showed a higher sensitivity (82% vs. 92%) and accuracy (79% vs. 72%) than conventional US [64]. In another study using SDUS, 76% of metastatic lymph nodes demonstrated peripheral vascularity and 85% of metastatic lymph nodes in patients with papillary thyroid carcinoma had maximum RI and PI values of 0.8 and 1.6, respectively [65]. Sometimes, parathyroid lesions can be mistaken for metastatic lymph nodes [66], but parathyroid adenoma shows a characteristic polar vessel sign in addition to its characteristic location [67]. In summary, central hilar vascularity is a typical finding of benign lymph nodes, but peripheral vascularity on Doppler US is an important finding with acceptable diagnostic performance for identifying metastatic lymph nodes in patients with thyroid cancer.
Summary, consensus, and limitations
Marked internal vascularity on CDUS and SMI may be a major finding of malignant thyroid nodules [23-30,54-56]. The emerging modality of SMI can reveal more vascular details than CDUS or PDUS [33-35]. However, the populations in previous studies had a wide range of prevalence of papillary thyroid carcinomas (63.3%-90.6%) [16,44-46]. The parameters of SDUS, including PI and RI, may be important in differentiating benign from malignant thyroid nodules [28,29,31,38,39]. Hypoenhancement and heterogeneous enhancement on CEUS may be major features of malignant thyroid nodules [44-49]. Peripheral vascularity on Doppler US is a major finding for diagnosing metastatic cervical lymph nodes in patients with thyroid cancer [8,62].
Although many studies have reported that Doppler US is useful for the differentiation of thyroid nodules, few studies have evaluated the added value of Doppler US over gray-scale US for the differentiation of thyroid nodules [55]. Future studies on the added value of Doppler US is necessary for risk stratification and biopsy decision-making. Additionally, there are regional disproportions in studies and many studies in the literature had low-quality designs (small numbers of patients and retrospective designs). Further studies are required on the use of Doppler US for the prediction of malignant thyroid nodules.
Diagnosis of Diffuse Parenchymal Disease of the Thyroid Gland
Although clinical and laboratory findings play a more substantial role in the diagnosis and treatment of diffuse thyroid disease (DTD), thyroid US makes an important contribution to the diagnosis of DTD [9-11]. The known gray-scale US features of DTD include increased or decreased parenchymal echogenicity, coarse echotexture, increased or decreased anteroposterior (AP) diameter of the thyroid, and the presence of marginal nodularity. Specific US findings for Graves disease (GD) and Hashimoto thyroiditis (HT) are thyroid inferno and micronodulation, respectively [68-72]. DTD can increase or decrease the vascularity of the thyroid gland, although differences in the extent of fibrosis and inflammation can also affect any measured values of vascularity [73-76].
Doppler US of the thyroid gland is helpful for the early detection of incidental asymptomatic DTD [69,77-79] and the differential diagnosis of GD with destructive thyroiditis in thyrotoxicosis [73-76,80-86]. It can also provide valuable information on the functional status of the thyroid and can be used to evaluate disease remission, recurrence, and response to treatment [68,73,81,84,87].
Differentiation of asymptomatic DTD from the normal thyroid gland
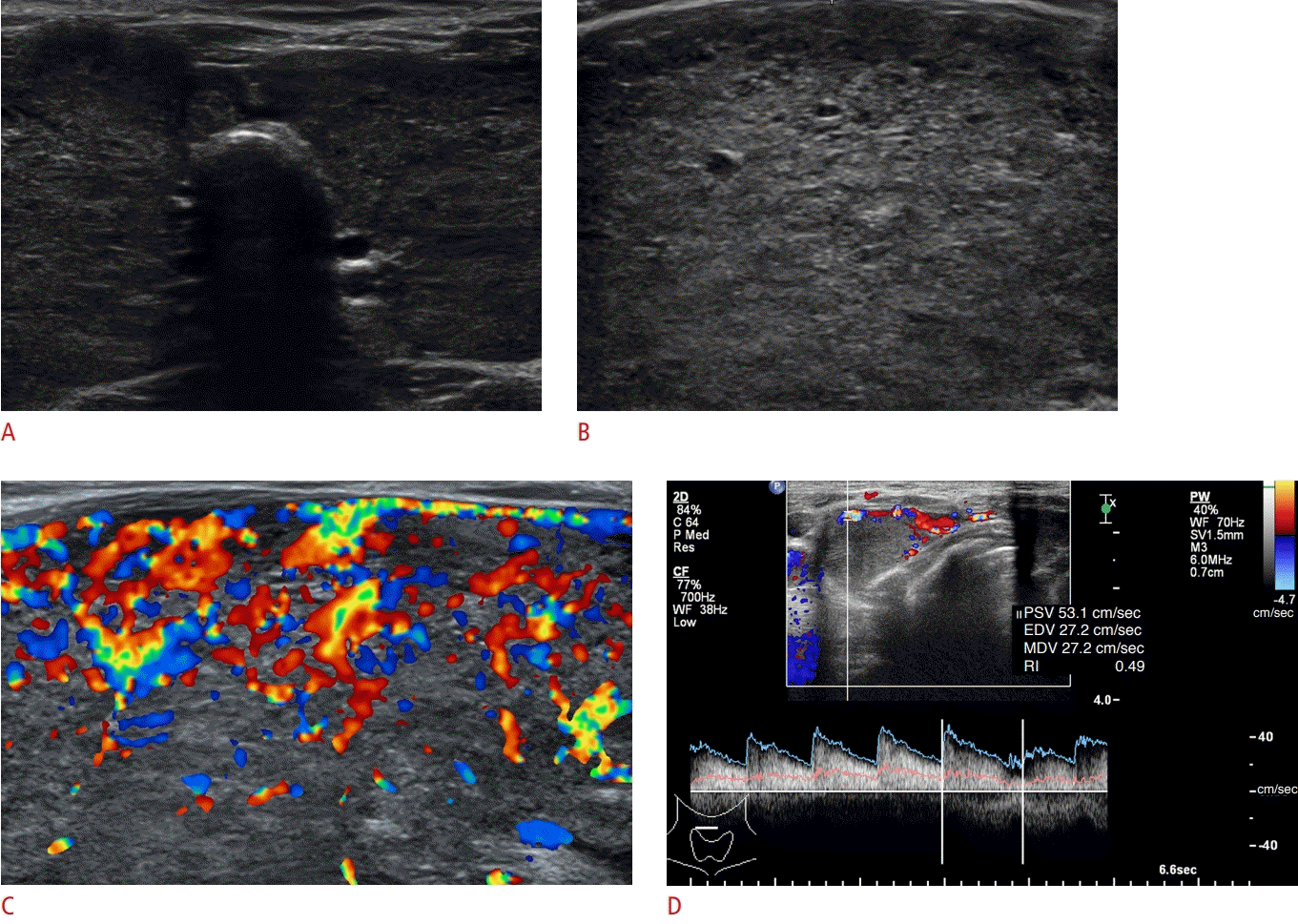
Thyroid US can be helpful in differentiating asymptomatic DTD from normal thyroid parenchyma [69,77-79]. A recent multicenter study found significant differences in US features of the thyroid gland between patients with normal thyroid parenchyma (n=139) and those with asymptomatic DTD (n=64; HT, non-HT, and diffuse hyperplasia; P<0.001); these differences included parenchymal echogenicity, parenchymal echotexture, AP diameter of the thyroid gland, glandular margin, and parenchymal vascularity. Several US features, including decreased or increased parenchymal echogenicity, coarse parenchymal echotexture, increased AP diameter (>2 cm), lobulated glandular margin, and increased parenchymal vascularity, were independent predictors of DTD (Fig. 4). The diagnostic ability of US was highest when a criterion of one or more of these abnormal US features were chosen, with a sensitivity of 85.9% and specificity of 63.3% [77]. Similarly, several investigators have demonstrated that three or more abnormal US features indicated a high likelihood of incidental DTD, with a sensitivity of 87.7% and specificity of 92.1% [69] and that one abnormal US feature showed the highest diagnostic accuracy, with a sensitivity of 80.5% and specificity of 85.7% [78].

A 50-year-old woman with Hashimoto thyroiditis diagnosed as diffuse thyroid disease on sonography.
Transverse (A) and longitudinal (B) gray-scale sonography show decreased echogenicity, coarse echotexture, a normal anteroposterior diameter of the thyroid gland, and lobulated margins. Longitudinal color Doppler sonography (C) shows increased parenchymal vascularity of the thyroid.
Gray-scale US can provide the volume of the thyroid gland and indicate features of DTD. The thyroid gland volume can be obtained from maximum measures in the longitudinal (L), AP, and transverse (T) axes of both lobes and the isthmus using the ellipse correction coefficient (thyroid volume=L×AP×T×π/6) [84]. In addition, unusual focal lesions found in patients with DTD need to be evaluated according to their features on B-mode and Doppler US and an investigation by fine-needle aspiration (FNA) biopsy may be indicated because papillary thyroid carcinoma and primary thyroid lymphoma are more likely in patients with HT than in the general population [88].
Differential diagnosis of GD from destructive thyroiditis in thyrotoxicosis
Doppler US is useful for the differential diagnosis of GD from destructive thyroiditis, which causes thyrotoxicosis in its early stage. Destructive thyroiditis includes HT, subacute granulomatous thyroiditis, postpartum thyroiditis, and painless (silent) thyroiditis [73-76]. Before treatment or effective therapy, GD shows a diffuse increase in vascularization of the parenchyma, referred to as thyroid inferno, a term first used by Ralls et al. in 1988 [68]. Thyroid hypervascularization can also occur in HT, but to a lesser degree (Fig. 5) [76,85,86]. The vascularity of the thyroid parenchyma can be determined using a visual scale according to the classification created by Schulz et al. [89]: pattern 0, blood flow limited to the peripheral thyroid arteries while parenchymal flow is absent; pattern I, presence of mildly increased parenchymal flow; pattern II, clearly increased color flow with a diffuse homogenous distribution; and pattern III, markedly increased color flow with a homogenous distribution.
A 31-year-old man with Graves disease diagnosed as diffuse thyroid disease on sonography.
Transverse (A) and longitudinal (B) gray-scale sonography show decreased echogenicity, coarse echotexture, an increased anteroposterior diameter of the thyroid gland, and smooth margins. A qualitative visual assessment of thyroid parenchymal vascularity on longitudinal color Doppler sonography (C, D) revealed thyroid inferno (C), while quantitative blood flow measurement revealed a peak systolic velocity (PSV) of 53.1 cm/sec in the right superior thyroid artery (D). EDV, end diastolic velocity; MDV, mean diastolic velocity.
Quantitative measurement of thyroid arterial blood flow is a useful parameter for the differential diagnosis of thyrotoxicosis. PSV measurements of the thyroid artery have become accurate and reliable with excellent reproducibility [75,83,86]. PSV measurement of thyroid arteries is performed using a 1- to 3-mm sample volume adjustment in the vessel center. The Doppler angle should be kept at or below 60° and the correction angle should be adjusted parallel to the direction of flow [75,83,86]. There is generally no substantial difference between PSV measurements made on the superior or inferior thyroid arteries or on the inferior thyroidal arteries of either side [76,80-82,86]. The cutoff value for differentiating GD from thyroiditis is 40-50 cm/sec [76,82,83,86]. In a study by Donkol et al. [76] on the differential diagnosis of GD and HT, Doppler US demonstrated a sensitivity of 88.9%, a specificity of 87.5%, a positive predictive value of 94.1%, a negative predictive value of 77.8%, and a diagnostic accuracy of 88.5% compared the reference standard of 99mTc scintigraphy. Similarly, in a study by Hari Kumar et al. [85], Doppler US had a sensitivity of 96% and specificity of 95% for the differential diagnosis of thyrotoxicosis in reference to 99mTc scintigraphy. Other methods of estimating thyroid blood flow, such as thyroid blood flow area, vascularization index, and highresolution power Doppler, have also been used to provide better differentiation [68,84,90].
Evaluation of disease remission, recurrence, and response to treatment in DTD
In patients with GD, thyroid gland vascularization correlates with the underlying functional status, and this vascularization decreases when the disease is under control, but it can increase in cases of recurrence. Many authors have reported that a decrease in vascularity occurs in parallel with biochemical remission and disease control in GD and suggested that thyroid Doppler US has the potential to monitor the therapeutic response in patients with GD [68,73,81,84,87]. In addition, patients who responded to treatment with drugs or radioiodine presented a significant reduction in parenchymal vascularity and the PSV of the inferior thyroidal artery, while patients who did not receive treatment or had no response to treatment showed no decrease in the PSV of the inferior thyroidal artery [73,81,87].
Previous studies have shown that Doppler US is useful for evaluating disease activity in HT and subacute granulomatous thyroiditis. In the early stages of disease, HT shows diffuse hypervascularization, which can be similar to the thyroid inferno described for GD, but in a less intense form and with a lower PSV in the thyroid arteries (<40 cm/sec) [75,76,85,86]. In the latter stages of HT, thyroid vascularity can decrease because of extensive fibrosis. However, the PSV values were significantly higher in HT patients with hypothyroidism than in their euthyroid counterparts [89], and the thyroid blood flow did not correlate with the functional state of the gland in HT [73,89]. Unlike GD or HT, subacute granulomatous thyroiditis shows decreased or scant vascularity in the acute stage and slightly increased vascularity in the recovery stage [91,92].
Summary, consensus, and limitations
Doppler US is a widely used method that may have comparable diagnostic performance to thyroid scintigraphy and does not appear to have contraindications [76,85]. Doppler US may be helpful for the early detection of incidental asymptomatic DTD and useful for the differential diagnosis of thyrotoxicosis, especially in the differentiation of GD from HT. In addition, Doppler US can be used to evaluate disease remission, recurrence, and response to treatment in DTD. However, it is dependent on a skilled operator [74,77,79]. Doppler US should be performed using sensitive equipment by a trained operator with good reproducibility [74,77,79]. It is important to use the proper technique with the appropriate transducer and equipment adjustments [74-77,79,83,86]. To improve clarity, further studies and guidelines regarding the standard Doppler US technique and its clinical applications are needed.
Interventional Procedures (Biopsy and Ablation)
US-guided thyroid biopsy
In US-guided thyroid biopsy, Doppler US can provide information for vessel mapping along the approach route of the biopsy needle and can detect vascular complications. Doppler US can provide information on blood vessels in and around the nodule during US-guided biopsy (FNA or core needle biopsy). To ensure a safe biopsy and minimize vessel injury, it is necessary to use Doppler US to completely map the vessels along the approach route (from the skin to the nodule). CDUS and SDUS provide information that allows for the differentiation of arteries and veins. After complete vessel mapping, the operator decides on the safe approach route and biopsy method to avoid the biopsy needle puncturing perithyroidal vessels during the US-guided biopsy procedure [3-7,13,93] (Fig. 6). Doppler US also helps to detect vascular complications, including pseudoaneurysms, which may result from needle-induced mechanical injury to the superior or inferior thyroid artery.

Ultrasound (US)-guided core needle biopsy of a thyroid nodule.
A. Before the procedure, the vessels along the approach route were carefully evaluated by Doppler US. B. Using a freehand technique, a core needle was directed from the isthmus toward the nodule while avoiding the vascular structures. C. After the tip of the biopsy needle was advanced into the edge of the nodule, the stylet and cutting cannula of the needle were sequentially fired.
US-guided thyroid ablation
In the US-guided ablation of thyroid nodules, Doppler US can be used for the following purposes: (1) pre-procedural evaluation of nodule vascularity, (2) vessel mapping along the approach route of the procedure device, and (3) post-procedural surveillance.
In a pre-procedural US examination with Doppler US, the size, proportion of solid components, vascularity, and internal contents of each nodule should be carefully evaluated according to the guidelines for radiofrequency ablation (RFA) and the consensus statement for ethanol ablation (EA) [3,4,7]. Doppler US provides information on the internal vascularity, feeding artery, and draining vein of each nodule (Fig. 7). Using this information, the RFA operator can decide on the appropriate treatment strategy, including the ablation method, number of treatment sessions, type of electrode, and technique (including artery-first ablation and marginal venous ablation) [4,7,94].
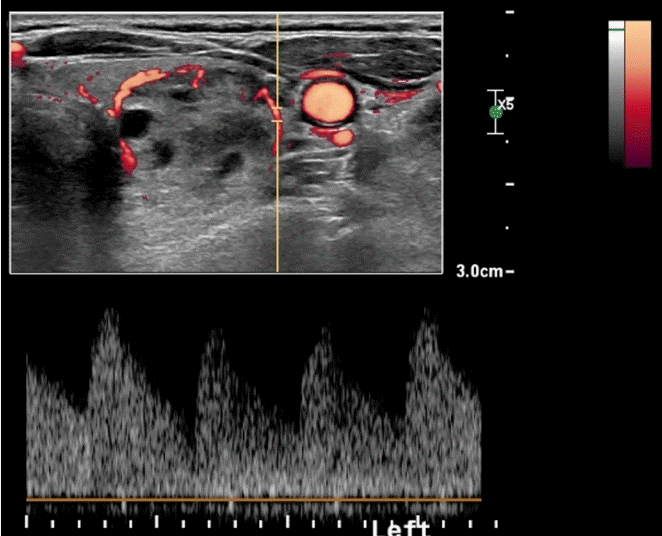
Feeding artery of a thyroid nodule.
Before thyroid radiofrequency ablation, the main feeding artery from the superior thyroid artery is identified on spectral Doppler ultrasonography.
Doppler US can also provide information for vessel mapping along the approach route to avoid vessel damage [4,7,94]. Hematomas, caused by mechanical injury to vessels from procedural devices including the electrode or needle, can develop in perithyroidal, subcapsular, and intranodular locations. To prevent hematomas, perithyroidal vessels, including the superior and inferior thyroid arteries, should be carefully evaluated using Doppler US before inserting procedural devices [4,7,94].
After EA and RFA procedures, Doppler US plays an important role in surveillance in addition to clinical symptoms. Doppler US is primarily used to detect any under-ablated portions of a lesion (Fig. 8). If a viable nodule portion with vascularity is detected on gray-scale US and CDUS in addition to persistent symptomatic and cosmetic problems, additional ablation is indicated [3,4,7,95,96]. This is because the under-ablated portion with vascularity has considerable potential for regrowth on follow-up [97]. However, CDUS does not have sufficient sensitivity to detect small vessels and slow blood flow [98]. To overcome these disadvantages of CDUS, some authors have suggested that CEUS can be used as an ancillary diagnostic tool for detecting under-ablated portions after an RFA procedure [99-101]. Doppler US is also a primary imaging modality during follow-up after other ablation procedures, including laser ablation [102-104], microwave ablation [105-107], and high-intensity focused US ablation [108,109]. Most studies have used a color Doppler technique [102,104-107,109], while some have used PDUS [108], SMI [103], or CEUS [103]. Some researchers have suggested that the ablation zone is clearer with CEUS than with CDUS [110,111]. Furthermore, a recent study has suggested that the accuracy of SMI for the detection of undertreated areas after laser ablation of thyroid nodules is similar to that of CEUS [103].
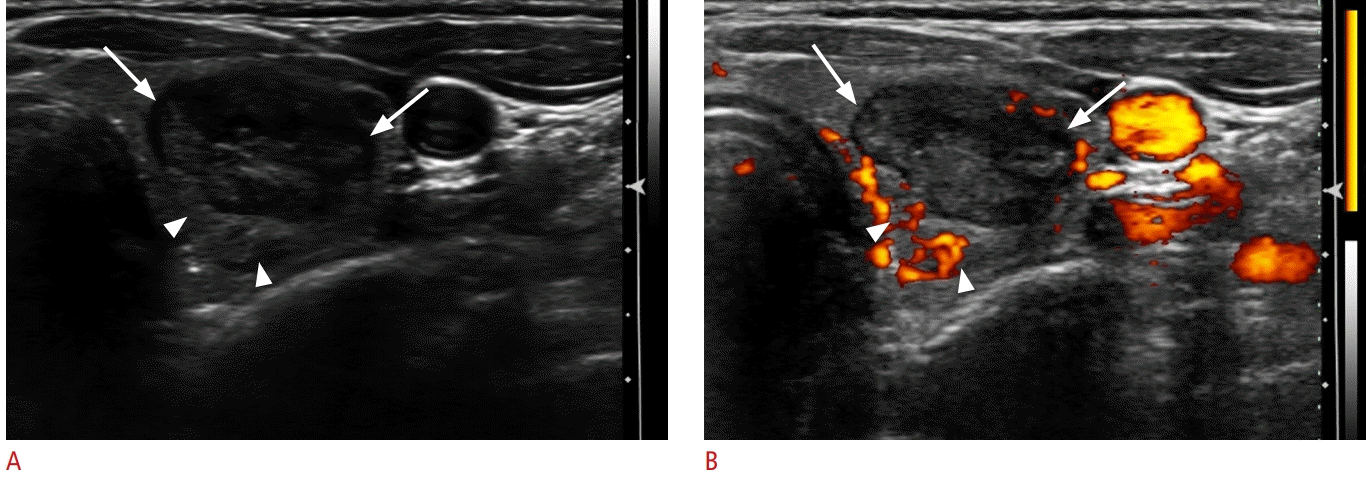
An under-ablated nodule portion on Doppler ultrasonography.
On gray-scale (A) and Doppler ultrasonography (B), most of the nodule shows low echogenicity without vascularity (arrows), thereby suggesting an ablated portion, but the medial-posterior portion of the nodule appears isoechoic with vascularity (arrowheads), suggesting the presence of an under-ablated portion.
Summary, consensus, and limitations
In US-guided biopsy, Doppler US can provide information for vessel mapping along the approach route of the biopsy needle and can detect complications including vessel injury. For US-guided ablation, Doppler US can provide information for pre-procedural evaluation of nodule vascularity, vessel mapping along the approach route of the procedure device, and post-procedural surveillance.
Harms and Benefits
Doppler US is widely used to assist in the diagnosis of thyroid nodules, metastatic cervical lymph nodes in patients with thyroid cancer, and thyroiditis, as well as for the monitoring of thyroid interventions. Moreover, there is no risk of exposure to ionizing radiation. All Doppler US techniques are non-invasive, except CEUS. Therefore, the potential of CEUS-induced harm should be considered when CEUS is used.
Acceptability and Applicability
An evaluation of the domestic acceptability and applicability leads us to conclude that Doppler US is useful and reasonable for the diagnosis and monitoring of thyroid interventions. In South Korea, the Korean National Insurance Service covers the cost of US, with no additional fee for CDUS, PDUS, SDUS, or SMI. However, CEUS is less acceptable because it is an invasive technique and the cost is not covered by national insurance. In future, the optimum US method for each task should be validated.
Conclusions and Future Perspectives
The Korean Society of Thyroid Radiology presents this first consensus statement on thyroid Doppler US to improve the diagnostic performance of thyroid nodules and ensure the safety and efficacy of thyroid interventions in daily practice. Considering the increasing use of thyroid interventions in the management of thyroid lesions, operators should have a good knowledge of thyroid Doppler US. However, the high-level evidence for thyroid Doppler US is insufficient, and future studies with high evidence levels are necessary to establish future thyroid Doppler US guidelines.
Notes
Author Contributions
Conceptualization: Choi YJ, Ha EJ, Baek JH, Na DG. Data acquisition: Chung J, Lee YJ, Choi YJ, Suh CH, Choi M. Data analysis or interpretation: Chung J, Lee YJ, Choi YJ, Suh CH, Choi M. Drafting of the manuscript: Chung J, Lee YJ, Choi YJ, Ha EJ, Baek JH, Na DG. Critical revision of the manuscript: Ha EJ, Baek JH, Na DG. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported and funded by the Korean Society of Radiology.