Erratum: Noninvasive assessment of hepatic steatosis using a pathologic reference standard: comparison of CT, MRI, and US-based techniques
Article information
Ultrasonography 2022;41:344-354
https://doi.org/10.14366/usg.21150
The authors and publisher of the article would like to bring the reader’s attention to an error in Table 2 on page 350.
The ATI data for ‘Diagnostic performance of imaging tools for detecting HS ≥5%’ is incorrect and should be revised. Please see the corrected Table 2 below for the accurate data.
Table 2.
Diagnostic performance of imaging tools for detecting HS ≥5%
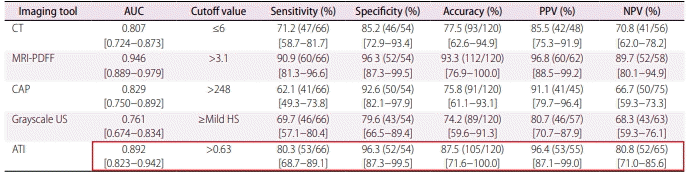
Values are percentages, with numerators and denominators in parentheses and 95% confidence intervals in brackets.
HS, hepatic steatosis; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; CT, computed tomography; MRI-PDFF, magnetic resonance imaging-derived proton density fat fraction; CAP, controlled attenuation parameter; US, ultrasound; ATI, attenuation imaging.