Ultrasound-guided ethanol ablation versus the Sistrunk operation as a primary treatment for thyroglossal duct cysts
Article information
Abstract
Purpose
This study compared ethanol ablation (EA) with the Sistrunk operation (SO) with regard to feasibility, treatment efficacy, and cost-effectiveness. The goal was to evaluate whether EA could replace SO as a primary treatment modality for thyroglossal duct cysts (TGDCs).
Methods
This retrospective case-control study included patients with TGDCs who were treated with either EA or SO between 2016 and 2022. The primary outcome variables evaluated were treatment efficacy (as measured by the volume reduction rate [VRR] and treatment success rate), complications, and cost-effectiveness.
Results
A total of 72 patients were enrolled, with 33 in the EA group and 39 in the SO group. The procedure or operation times for the EA and SO groups were 9 and 82 minutes, respectively (P<0.001). At the final follow-up appointment, the VRR was 94.1% for the EA group and 100.0% for the SO group (P<0.001). Treatment success was achieved for 32 patients (97.0%) in the EA group and for all 39 patients (100.0%) in the SO group (P=0.458). The overall complication rates were 0.0% and 17.9% in the EA and SO groups, respectively (P=0.013). The total costs, including all treatment procedures and follow-up ultrasound examinations, were $485 and $1,081.7 for the EA and SO groups, respectively (P<0.001).
Conclusion
EA demonstrates superiority over SO in terms of feasibility, safety, and costeffectiveness, while maintaining comparable treatment efficacy. Despite the need for multiple treatment sessions in approximately one-quarter of patients, EA can serve as a primary treatment modality for selected patients with TGDCs, supplanting SO.
Introduction
Thyroglossal duct cysts (TGDCs), which are remnants of the thyroid anlage that arise during the embryonic development of the thyroid gland, are the most common congenital neck masses [1]. The current standard treatment for TGDCs is the Sistrunk operation (SO), which was introduced in 1920. This procedure involves the complete excision of the TGDC, along with a central portion of the hyoid bone [1-3]. While the SO has markedly improved the management of TGDCs by reducing the recurrence rate from 45%-55% to 1%-5% compared to simple excision, it is accompanied by several surgical burdens. These include the requirement for general anesthesia, visible surgical scarring on the neck, and the potential for postoperative complications [2-4].
To overcome the drawbacks of surgery, Fukumoto et al. first attempted ultrasound (US)-guided ethanol ablation (EA) (US-EA) for TGDCs in 1994, suggesting the potential of US-EA as a primary treatment for TGDC [5]. Since then, numerous studies have explored the efficacy and safety of US-EA for TGDCs, reporting high rates of volume reduction and treatment success without major complications [6-9]. An early study on EA for TGDCs, involving 11 patients, reported a mean volume reduction rate (VRR) of 81.3% and a treatment success rate of 80.0% following EA [8]. A more recent study of 28 patients yielded a mean VRR of 95.1% and demonstrated a significant improvement in cosmetic scores [7]. In 2021, a systematic review was published that included 82 patients from four studies on EA for TGDCs, reporting a pooled success rate of 84% [6]. In addition to these promising results, US-EA offers distinct advantages as an office-based technique that does not require general anesthesia, as well as a minimally invasive procedure without surgical scarring.
Despite the promising research and potential advantages, US-EA is not yet recognized as a primary treatment modality for TGDCs due to the limited number of studies and data available compared with SO, the current standard treatment. Indeed, since 2000, fewer than 10 studies have been published on the use of EA for TGDCs, only one of which compared the outcomes of EA with those of surgery [6-11].
Therefore, the present study was conducted to compare the feasibility, treatment efficacy, and cost-effectiveness of EA and SO, with a focus on evaluating EA to replace surgery as a primary treatment for TGDCs.
Materials and Methods
Compliance with Ethical Standards
The protocol for this retrospective case-control study was approved by the institutional review board of the authors’ institution Kyungpook National University Hospital (Registration No., 202303002), and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Given the retrospective nature of the study, the requirement for written informed consent from patients was waived.
Patients
The inclusion criteria encompassed patients with TGDCs who underwent treatment with either EA or SO between 2016 and 2022. Because not all TGDCs are clinically meaningful, treatment was indicated only for cases involving TGDC-related discomforts, such as cosmetic deformity, a sense of pressure, and swallowing difficulties, or TGDC-related complications, such as recurrent infection and cutaneous fistula. The exclusion criteria were as follows: (1) TGDC diagnosed as malignant (TGDC carcinoma) on permanent pathological examination after surgery, (2) a history of surgery or irradiation for head and neck cancer, and (3) a follow-up period of less than 6 months after EA or SO.
Preprocedural Evaluation
All patients underwent US and computed tomography examinations to evaluate the characteristics of the TGDC. The volume of the TGDC was calculated using the following equation: volume=π(abc)/6, in which ‘a’ represents the transverse diameter, ‘b’ the anteroposterior diameter, and ‘c’ the craniocaudal diameter. Furthermore, US-guided fine-needle aspiration cytology was employed to diagnose a benign TGDC and to rule out malignancy. Either HS 70A (Samsung Medison, Seoul, Korea) or Aplio i700 (Canon Medical Systems, Tochigi, Japan) US machines were used for all procedures. Liquidbased cytology (Thin-Prep) was utilized for cytological evaluation. In certain patients, thyroglobulin levels were measured in the washout fluid from the fine-needle aspiration cytology needle to distinguish thyroid follicle-containing lesions (benign TGDC or TGDC carcinoma) from other types of lesions (dermoid or lymph node).
Decision of Treatment Modality
Prior to the determination of treatment modality, the advantages and disadvantages of each option were discussed. Primary EA was recommended for the following indications: (1) patients aged 18 years or older with TGDCs, (2) those with benign TGDCs on imaging and cytological examinations, and (3) those who preferred a non-surgical, scarless treatment for TGDCs. In contrast, primary SO was recommended for the following indications: (1) patients under 18 years old with TGDCs, (2) those who preferred surgery over EA, especially as a single-stage treatment, (3) cases involving any suspicious findings of possible malignancy, and (4) cases of TGDCs accompanied by cutaneous fistula.
SO and Follow-up
A transverse cervical incision was made at the level of the mass, and the skin flap was elevated in the subplatysmal plane. The strap muscles were divided in the midline through the raphe, and the mass was meticulously dissected toward the body of the hyoid bone. The mass was then removed along with the lateral thirds of the hyoid bone. Patients were scheduled for follow-up appointments at 0.5, 3, and 6 months after surgery, and annually thereafter. Follow-up US was not performed as a matter of routine, but rather was utilized only when postoperative seroma or recurrence was suspected based on physical examination.
EA and Follow-up
The patients were placed in the supine position with the neck extended. Local anesthesia was not typically administered, as the EA procedure is generally completed with a single needle puncture and TGDCs lack sensory innervation. Under US guidance, a 21- to 23-gauge needle was inserted into the cyst to aspirate its contents (Fig. 1A). Following aspiration, 99% sterile ethanol was injected into the cystic cavity using the same needle until the collapsed cyst began to re-expand (Fig. 1B). The volume of ethanol used in a single treatment session generally did not exceed 2.5 mL. However, if the volume of aspirate was greater than 20 mL, 5-7 mL of ethanol was injected. Following ethanol injection, the needle was left in place for 1-2 minutes to prevent immediate leakage of the injected ethanol. The needle was then removed, and the puncture site was lightly compressed for 10 minutes. At the conclusion of the EA procedure, the injected ethanol was not aspirated but was instead retained within the TGDC. This was based on the presumption that retaining the ethanol would yield superior treatment outcomes, even with a small amount of ethanol, as suggested by several studies on EA for cystic thyroid nodules [12-14]. For TGDCs with viscous contents that could not be fully aspirated, approximately 1 mL of ethanol was injected during the initial EA session to liquefy the contents. A second EA session was then scheduled within a 1- to 2-month interval.
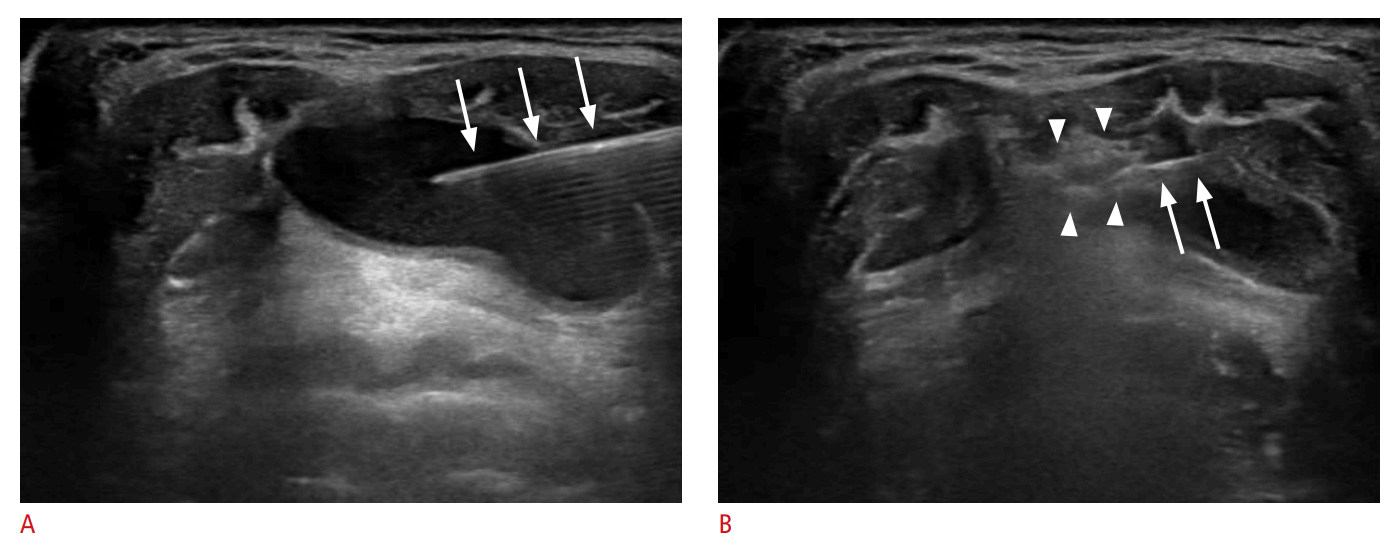
Ultrasound-guided ethanol ablation procedure.
A. After the insertion of a 21-gauge needle (arrows) into the cyst, the internal content was aspirated. B. Following aspiration, 99% sterile ethanol was injected into the cystic cavity using the same needle (arrows). The injected ethanol presents as a hyperechoic flush (arrowheads) on real-time ultrasound.
The ablated TGDCs were evaluated using follow-up US at 2, 4, 6, and 12 months after EA and every 12 months thereafter. If US examination revealed cystic remnants in the ablated TGDC, or if TGDC-related discomfort persisted during follow-up despite successful treatment, an additional EA session was considered. Regrowth of the ablated mass was defined as an increase of more than 20% from the last recorded TGDC volume, as identified in two consecutive follow-up US examinations. This cutoff value was established based on the observation that even a minor volume increase in a TGDC can lead to noticeable cosmetic deformities. This is because TGDCs, unlike thyroid nodules, are not surrounded by normal parenchyma and are located more superficially [15].
Assessment Parameters and Statistical Analyses
The primary outcome variables evaluated were the treatment efficacy, complications, and cost-effectiveness of EA and SO. Treatment efficacy was assessed using the VRR, calculated as ([initial volume–final volume]/initial volume×100%), and treatment success. A VRR of ≥70% was used as the cutoff value for defining success, rather than a VRR of ≥50%, to avoid overestimating the treatment efficacy of EA compared to SO [10,16]. For the SO group, the VRR was considered to be 100% if the TGDC was completely excised during surgery. Cost-effectiveness was evaluated based on the total costs for EA and SO, which were estimated by adding the cost of each treatment session to the cost of follow-up US examinations. The secondary outcome variables included procedure time (from needle insertion to withdrawal) or operation time (from the initiation to the end of general anesthesia), hospital stay duration, number of treatment sessions, World Health Organization (WHO) cosmetic score, and complications. All variables were compared between the EA and SO groups. For EA, a subgroup analysis was conducted according to treatment success after a single session of EA to identify potential risk factors associated with the need for multiple treatment sessions to achieve success.
SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Continuous data were presented as the median (interquartile range [IQR]). To evaluate differences between the groups, the chi-square test was used for categorical data and the Mann-Whitney U test for continuous data. Statistical significance was considered to be indicated by a two-sided P-value of less than 0.05.
Results
Baseline Patient Characteristics
Between 2016 and 2022, 99 patients with TGDC were managed at this institution. Of these, 33 patients who underwent EA and 39 who underwent SO met the inclusion criteria. The baseline characteristics of these patients are presented in Table 1. No significant differences were observed in terms of age, sex, comorbidity, initial clinical presentation, history of infection, or initial WHO cosmetic score. The median age for the EA group was 41 years (IQR, 31.5 to 58.0 years), while it was 47 years (IQR, 32.0 to 56.0 years) for the SO group. In the EA group, 19 patients (57.6%) were male and 14 (42.4%) were female, while in the SO group, 19 patients (48.7%) were male and 20 (51.3%) were female. No significant differences were observed in age, sex, comorbidity, initial clinical presentation, history of infection, or initial WHO cosmetic score.
Baseline TGDC Characteristics
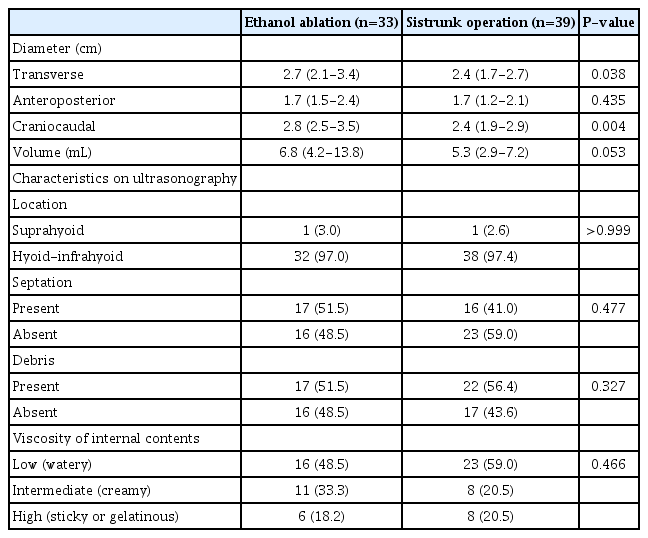
Table 2 presents the baseline characteristics of the TGDCs. In the EA and SO groups, the transverse diameter measurements were 2.7 cm (IQR, 2.1 to 3.4 cm) and 2.4 cm (IQR, 1.7 to 2.7 cm), respectively (P=0.038). The anteroposterior diameter values were 1.7 cm (IQR, 1.5 to 2.4 cm) and 1.7 cm (IQR, 1.2 to 2.1 cm), respectively (P=0.435), and the craniocaudal diameter measurements were 2.8 cm (IQR, 2.5 to 3.5 cm) and 2.4 cm (IQR, 1.9 to 2.9 cm), respectively (P=0.004). The initial TGDC volumes were 6.8 mL (IQR, 4.2 to 13.8 mL) and 5.3 (IQR, 2.9 to 7.2 mL) in the EA and SO groups, respectively (P=0.053). No significant differences were observed between groups in several US characteristics, including location, septation, debris, and viscosity of the internal contents.
Feasibility and Outcomes
In the EA and SO groups, the median procedure or operation times were 9 minutes (IQR, 7.5 to 10.0 minutes) and 82 minutes (IQR, 70.0 to 95.0 minutes), respectively (P<0.001) (Table 3). The median hospital stay durations were 0 days (IQR, 0 to 0 days) and 4 days (IQR, 3 to 5 days), respectively (P<0.001). For the first EA session, the median volumes of aspirate and injected ethanol were 3.0 mL (IQR, 2.0 to 8.0 mL) and 2.0 mL (IQR, 1.3 to 2.0 mL), respectively, with mean values of 6.6±9.9 mL and 2.1±1.2 mL, respectively. In the EA group, the total number of treatment sessions was one for 19 patients (57.6%), two for 10 patients (30.3%), three for three patients (9.1%), and four for one patient (3.0%). In the SO group, it was one for 38 patients (97.4%) and two for one patient (2.6%). The difference between groups in the total number of treatment sessions was statistically significant (P=0.001). The median follow-up periods in the EA and SO groups were 26 months (IQR, 12 to 37 months) and 23 months (IQR, 12 to 40 months), respectively (P=0.791). The median VRR values at the last follow-up session were 94.1% (IQR, 87.8% to 100.0%) and 100.0% (IQR, 0 to 0) in the EA and SO groups, respectively (P<0.001) (Fig. 2). Treatment success was achieved in 32 (97.0%) and 39 (100.0%) patients in the EA and SO groups, respectively (P=0.458). The cumulative treatment success rates after the first, second, third, and fourth EA sessions were 75.8% (25 of 33 patients), 90.9% (30 of 33), 93.9% (31 of 33), and 97.0% (32 of 33), respectively. During follow-up, no TGDC mass regrowth was observed in the EA group, while one patient in the SO group experienced recurrence and underwent revision surgery in a second treatment session. At the final follow-up appointment, the WHO cosmetic score was 1 for 32 (97.0%) patients and 3 for one (3.0%) patient in the EA group (Fig. 3). In the SO group, the WHO cosmetic score was 1 for all patients. The difference in WHO cosmetic scores between the groups was not statistically significant (P=0.458).

Ultrasound (US) findings of a thyroglossal duct cyst before and after ethanol ablation.
A. A cystic mass of approximately 3.0 cm×1.8 cm was identified on initial transverse US of the anterior neck. B. The mass had completely disappeared on follow-up US 4 months after a single session of ethanol ablation, constituting a 100% volume reduction rate.

The external neck with a thyroglossal duct cyst before and after ethanol ablation.
A. A mass is easily visible in the upper anterior neck region, with a World Health Organization (WHO) cosmetic score of 4. B. Two months after a single session of ethanol ablation, no visible or palpable mass was identified, constituting a WHO cosmetic score of 1.
Clinicodemographic Characteristics According to Treatment Success after the Initial EA Session
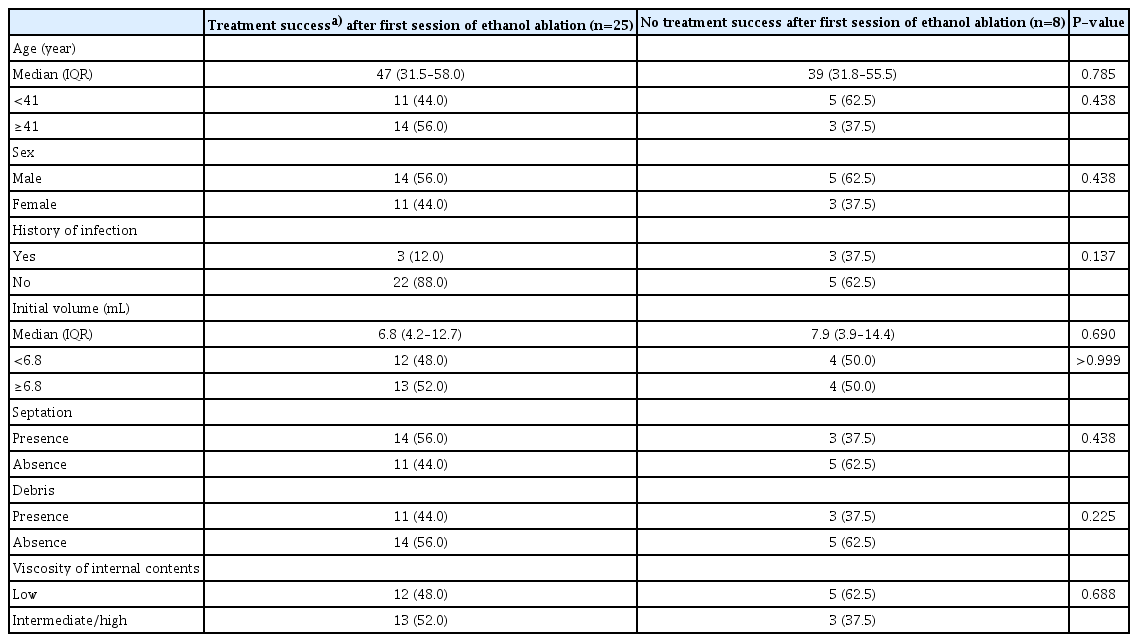
No significant differences were observed in clinicodemographic characteristics, including age, sex, history of infection, initial volume, septation, debris, and viscosity of the internal contents, according to treatment success after the first session of EA (Table 4).
Complications and Costs
No complications were observed in the EA group. In the SO group, the overall incidence of complications was 17.9% (seven of 39 patients). This included one case (2.6%) of wound infection, four cases (10.3%) of seroma, and two cases (5.1%) of hypertrophic/keloid scars. The difference in the incidence of overall complications between the groups was statistically significant (P=0.013) (Table 5).
The median total treatment costs for the EA and SO groups were $318.3 (IQR, $287.5 to $542.9) and $1,053.3 (IQR, $810.0 to $1,265.0), respectively (P<0.001). The median total costs of the US examinations conducted during the follow-up period were $166.7 (IQR, $166.7 to $250.0) for the EA group and $0 for the SO group, respectively (P<0.001). When considering the overall costs, including both treatment procedures and follow-up US examinations, the EA group incurred a median cost of $485 (IQR, $454.2 to $748.8), compared to $1,081.7 (IQR, $893.3 to $1,265.0) for the SO group (P<0.001) (Table 5).
Discussion
This study demonstrated that US-EA not only is more feasible, but also offers comparable treatment efficacy and superior cost-effectiveness relative to SO, the current standard treatment for TGDCs. These promising findings suggest that EA could potentially serve as a primary treatment for TGDCs.
EA is an office-based, minimally invasive technique that has been used to treat various benign cystic lesions, such as cystic thyroid nodules and branchial cleft cysts. Its feasibility has been well-documented in numerous previous studies [12,17-19]. In the present study on TGDCs, the EA procedures were successfully completed in a median time of 9 minutes, without the need for anesthesia or hospitalization. As such, EA may offer greater feasibility for the treatment of TGDCs compared to SO, for both patients and surgeons. This is due to the presence of fewer restrictions related to patient comorbidity, treatment time, and treatment location. However, EA is not suitable for complex TGDC cases, particularly those involving cutaneous fistulas, as it is impossible to retain the injected ethanol within the cyst. Furthermore, this technique may necessitate the use of general anesthesia in pediatric patients who are unable to cooperate during the procedure. Regarding complications, in the present study, a significantly lower complication rate was observed in the EA group compared to the SO group (0% vs. 17.9%, respectively), aligning with the results of an investigation published in 2017 [9]. As such, EA is considered to have a better safety profile than SO, further supporting the use of EA as a primary treatment for TGDCs.
The primary criterion for adopting a treatment modality should be efficacy. In this study, although the initial TGDC volume was greater in the EA group compared to the SO group (6.8 mL vs. 5.3 mL, P=0.053), the VRR of the EA group at the last follow-up examination was 94.1%. In addition, the treatment success rate of EA (97.0%) was comparable to that of SO (100.0%), indicating acceptable efficacy of EA as a primary treatment modality for TGDCs. In fact, most previous studies have reported favorable treatment efficacy for EA, with VRRs ranging from 76.7%-95.1%, demonstrating the consistent efficacy of EA for TGDCs [6-11]. To date, only one study has compared EA with surgery in patients with TGDCs, reporting an 82.3% VRR and 85.7% treatment success rate following EA [9]. Although the treatment failure rate was higher for EA than for surgery (19.6% vs. 2.4%, P<0.001), the authors concluded that EA still offered acceptable treatment efficacy and a better safety profile than surgery [9].
Despite the promising treatment outcomes for EA, concerns have been raised about the short-lived therapeutic effect and the potential for regrowth of the ablated TGDC in the early follow-up period. This is because unlike surgery, EA does not completely eradicate the mass. In fact, few studies have provided information about the regrowth or recurrence of TGDCs after EA [11]. A 2021 study involving 28 patients reported no recurrence during a 12-month follow-up period [7]. Similarly, a 2017 study tracked 45 patients who had experienced successful treatment outcomes after EA, revealing that 88.8% of these patients retained their treatment success at the 1-year follow-up [9]. In the present study, no regrowth was observed in patients who exhibited treatment success after EA during a median follow-up period of 26 months, which is the longest follow-up duration among studies on EA for TGDCs. In addition, eight of the 32 patients who achieved treatment success underwent follow-up for more than 48 months, and none of these patients showed any regrowth of the ablated TGDC. This indicates that the therapeutic effect of EA can last for at least several years, and that early recurrence may not be a major concern when considering EA as a primary treatment for TGDCs. Furthermore, studies on EA for thyroid cysts have reported a VRR at 5-year follow-up of between 86.6% and 98.5%, with a recurrence rate as low as 3.1% to 18%. This suggests that EA could provide stable long-term efficacy in the treatment of TGDCs [12,18,20,21].
Although EA is highly feasible and offers satisfactory treatment efficacy for TGDC, it is not a single-stage treatment and may necessitate longer and more frequent treatment courses than surgery. Contrary to the single-stage surgical success achieved by SO in most patients, the success rate after the first EA session in this study was 75.8%. This means that approximately one-quarter of patients (24.2%) required multiple EA sessions to achieve treatment success. This proportion of patients requiring additional treatment sessions resembled that reported in previous research. In a 2011 study involving 11 patients with TGDCs, repeat EA was performed in 27% of participants [8]. Another relatively large-scale study reported that 26.8% of patients required multiple EA sessions [9]. Therefore, when considering EA as a primary treatment for TGDCs, physicians should thoroughly inform patients about the potential need for multiple treatment sessions and an extended overall treatment course. In the present study, although a subgroup analysis was conducted in an effort to stratify the risk of requiring multiple EA sessions to achieve treatment success, these authors found no patient or TGDC characteristics associated with the need for multiple sessions. This could primarily be due to the small number of cases in each subgroup. However, this finding could also suggest that EA can provide consistent treatment efficacy regardless of various patient and TGDC characteristics.
When considering a treatment approach in clinical practice, cost-effectiveness is a major consideration for both patients and physicians. In the context of EA for TGDCs, Chung et al. [9] were the first to address the treatment costs of EA compared to surgery, providing the only data currently available. In their study, the cost of EA (423,801 won, or approximately $353.1) was significantly lower than that of surgery (1,435,707 won, or approximately $1,200) (P<0.001) [9]. However, that previous study did not account for the total cost associated with multiple treatment sessions and follow-up US examinations throughout the full EA treatment course. As a result, the total costs of EA may have been underestimated. In the present study, the objective was to evaluate the true costs for each treatment modality by including the total costs for treatment sessions and follow-up US procedures. While the median cost for the total EA procedures ($318.3) was similar to that reported previously by Chung et al. [9] ($353.1), the overall cost increased when adding the costs for follow-up US scans. These are necessary to regularly assess treatment outcomes during the follow-up period after each EA session. However, the overall cost of EA remained significantly lower than that of surgery, at less than half of the surgical cost ($485 vs. $1,081.7). Therefore, EA was shown to be more cost-effective than SO as a primary treatment for TGDCs. However, in the case of four patients who underwent three or more sessions of EA, the overall cost of EA was comparable to or exceeded the median cost of SO. Therefore, EA may not have been cost-effective for these patients.
This study had several limitations. First, despite having the third largest sample size among all studies on EA for TGDCs, it may be insufficient to support EA as a standard treatment modality for TGDCs, completely supplanting SO. Second, indications for using EA and SO were not uniform, but were instead tailored to factors such as age, patient preferences, and clinical presentation. This issue should be considered when interpreting the study results. In particular, pediatric patients with TGDCs were assigned to the SO group due to concerns about the feasibility and safety of EA; thus, the use of EA as a primary treatment for pediatric patients could not be assessed. Third, although this study revealed a significantly lower overall cost compared to that of surgery, the cost of each treatment modality may vary greatly across countries. Fourth, due to the retrospective nature of the study, the cosmetic outcomes of surgical scars in the SO group could not be assessed. Therefore, the cosmetic superiority of the EA approach, which may be the most notable advantage of EA over SO, could not be objectively demonstrated.
With respect to the specific EA technique, two major issues related to this study require clarification: (1) the volume of ethanol injection and (2) the retention or aspiration of the injected ethanol at the conclusion of EA. A literature review of seven studies on EA for TGDC published since 2010 revealed that five studies referenced 50% or more of the aspirate volume as the minimum limit for ethanol injection (up to 15 mL of ethanol) [7-10,16,19,22]. However, in the current study, a relatively small volume of ethanol was used (≤2.5 mL). This decision was based on the hypothesis that the effective volume of ethanol depends not on the initial cyst volume or aspirate volume, but rather on the collapsed cyst volume following aspiration. This is because the therapeutic effect of EA is achieved through the exposure of the secretory lining cells of the cystic wall to ethanol [16]. While limited research is available on the appropriate volume of ethanol injection in EA for TGDCs, a study on EA for cystic thyroid nodules by Cho et al. in 2021 [23] reported a high treatment success rate (88.5%) even after a single EA session using a low dose of ethanol (≤5 mL). This suggests that EA with low-dose ethanol is effective regardless of the initial cyst volume and aspirate properties [23]. Second, the present study employed the retention method; this contrasts with several studies that used the aspiration method, which involves the aspiration of injected ethanol at the end of the EA procedure after a retention time of 5-10 minutes [7-10,16,19,22]. In fact, although no consensus exists on whether ethanol should be fully aspirated after instillation, these authors presumed that retention of the injected ethanol may be associated with better treatment outcomes, as the duration of the chemical reaction is longer than with the aspiration method [13,14]. However, since no individual studies have directly compared the retention and aspiration methods of EA for TGDCs with regard to treatment efficacy and complications, this technical variation remains at the discretion of the operator.
In conclusion, EA is a highly feasible option for the treatment of TGDCs, capable of being completed within 10 minutes and without surgical burdens such as general anesthesia and surgical scarring. Relative to SO, EA can offer comparable treatment efficacy, a superior safety profile, and superior cost-effectiveness, although this technique may require multiple treatment sessions to achieve success in approximately one-quarter of patients. Therefore, EA could serve as a primary treatment modality, replacing SO in select patients with TGDCs.
Notes
Author Contributions
Conceptualization: Ahn D. Data acquisition: Ahn D, Kwak JH, Lee GJ, Sohn JH. Data analysis or interpretation: Ahn D. Drafting of the manuscript: Ahn D, Kwak JH. Critical revision of the manuscript: Ahn D, Lee GJ, Sohn JH. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Key point
Ethanol ablation (EA) is a highly feasible option for the treatment of thyroglossal duct cysts (TGDCs) and can be completed within 10 minutes without surgical burden. EA offers a treatment efficacy comparable to that of the Sistrunk operation (SO), with a volume reduction rate of 94.1% vs. 100.0%; additionally, it demonstrates superior cost-effectiveness ($485 vs. $1,081.7). EA can serve as a primary treatment modality in select patents with TGDCs, thereby replacing SO.