Early quantification of the therapeutic efficacy of the vascular disrupting agent, CKD-516, using dynamic contrast-enhanced ultrasonography in rabbit VX2 liver tumors
Article information
Abstract
Purpose:
To evaluate the usefulness of dynamic contrast-enhanced ultrasonography (DCE-US) in the early quantification of hemodynamic change following administration of the vascular disrupting agent (VDA) CKD-516 using a rabbit VX2 liver tumor model.
Methods:
This study was approved by our institutional animal care and use committee. Eight VX2 liver-tumor-bearing rabbits were treated with intravenous CKD-516, and all underwent DCE-US using SonoVue before and again 2, 4, 6, and 24 hours following their treatment. The tumor perfusion parameters were obtained from the time-intensity curve of the DCE-US data. Repeated measures analysis of variance was performed to assess any significant change in tumor perfusion over time. Relative changes in the DCE-US parameters between the baseline and follow-up assessments were correlated with the relative changes in tumor size over the course of seven days using Pearson correlation.
Results:
CKD-516 treatment resulted in significant changes in the DCE-US parameters, including the peak intensity, total area under the time-intensity curve (AUCtotal), and AUC during wash-out (AUCout) over time (P<0.05). Pairwise comparison tests revealed that the AUCtotal and AUC during wash-in (AUCin) seen on the two-hour follow-up were significantly lower than the baseline values (P<0.05). However, none of early changes in the DCE-US parameters until 24-hour follow-up showed a significant correlation with the relative changes in tumor size during seven days after CKD-516 treatment.
Conclusion:
Our results suggest that a novel VDA (CKD-516) can cause disruption of tumor perfusion as early as two hours after treatment and that the therapeutic effect of CKD-516 treatment can be effectively quantified using DCE-US.
Introduction
Tumor neovascularization is a critical step for tumor growth and results in structurally and functionally abnormal tumor blood vessels, which are then worthwhile targets for anti-cancer treatment [1,2]. Recently, anti-vascular therapy has been widely investigated as a promising approach for cancer treatment [3-5]. According to the action mechanism, anti-vascular drugs can be divided into two categories, that is, an anti-angiogenic agent which inhibits the outgrowth of new vessel formation from pre-existing vessels, and a vascular disrupting agent (VDA) which destroys the established tumor vessels [6].
Monitoring the therapeutic efficacy of anti-vascular therapy as well as the early prediction of tumor response is of great importance, as it may quicken making a go or no-go decision for each patient, which will maximize the benefits and minimize the drawbacks of treatment [7]. Although tumor size change has traditionally been used to assess the cancer treatment effects of chemotherapy, size measurement may be insensitive or delayed chronologically during the monitoring of anti-vascular treatment and thus cannot be relied upon to accurately and promptly indicate the therapeutic effect [8]. Current studies have reported the usefulness of quantitative imaging methods including dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), DCE computed tomography (DCE-CT), and DCE ultrasound for monitoring the therapeutic effect of antivascular treatment, which can demonstrate hemodynamic changes noninvasively and longitudinally [3,6]. Among those imaging methods, DCE ultrasonography (DCE-US) has several advantages over DCE-MRI and DCE-CT, as it can be easily performed repeatedly at low cost and without patient exposure to ionizing radiation, and the ultrasound contrast agent is a purely intravascular marker of blood flow and perfusion that is not confounded by extravascular diffusion [7,9].
CKD-516 is a novel, small-molecule VDA which acts by inhibiting tubulin polymerization, causing rapid disruption of established tumor vessels by microtubule destabilization and cell apoptosis by cell-cycle arrest [10]. CKD-516 is in the ongoing phase I clinical trial period; therefore, appropriate timing of imaging to determine its therapeutic efficacy is critical. A recent preclinical study using DCE-MRI revealed a significant decrease in the tumor perfusion parameters seen at the four-hour follow-up and a significant recovery seen at the 48- hour follow-up following the CKD-516 treatment [11]. However, no previous study has shown serial perfusion changes induced by CKD-516 using DCE-US or a correlation between the DCE-US parameters and the tumor response.
Therefore, this preclinical study using rabbit VX2 liver tumor models investigated the usefulness of DCE-US in the early quantification of hemodynamic changes seen serially after administration of a novel VDA (CKD-516) and investigated whether DCE-US parameters would be early predictors of the tumor response.
Materials and Methods
Animal Model and Experiment Schedule
This study was approved by the Animal Care and Use Committee of Seoul National University Hospital. Fourteen male New Zealand White rabbits weighing between 2.5 and 3.5 kg were used. Prior to tumor implantation, the animals were sedated by intravenous injection of 5 mg/kg of a 1:1 combination of tiletamine hydrochloride and zolazepam (Zoletil; Virbac, Carros, France) and xylazine hydrochloride (Rompun 2%; Bayer Korea, Seoul, Korea). Through a midline abdominal incision, the left lobe of the liver was exposed and an approximately 5-mm tunnel was created in the subcapsular area of the left lobe of the liver. Then, approximately 1-mm3 minced pieces of freshly harvested VX2 carcinoma tissue were locally implanted in the liver via the tunnel. The VX2 liver tumors were incubated for 10 to 15 days after tumor implantation and prior to the baseline imaging. Fourteen tumor-carrying rabbits were randomly divided into the CKD-516-treated group (n=8) and the control group (n=6). CKD-516 solution was administered to the treated group immediately following the baseline ultrasound imaging. For each rabbit of the CKD-516-treated group (n=8), follow-up DCE-US studies were performed at two, four, six, and 24 hours following CKD-516 administration. At the baseline and sevenday follow-up examinations, the longest tumor dimensions were measured on ultrasound. To evaluate the reproducibility of the DCEUS parameters, the control group rabbits (n=6) underwent repeated DCE-US studies at two-hour intervals, that is, at baseline and the two-hour follow-up.
Vascular Disrupting Agent (CKD-516) Preparation
CKD-516 (Chong Kun Dang Pharm, Seoul, Korea) is a novel watersoluble VDA which acts by inhibiting tubulin polymerization [12]. For each rabbit in the treated group, CKD-516 solution, which was prepared by dissolving CKD-516 at a dose of 9 mg/m2 body surface area in 5 mL of normal saline, was administered by slow intravenous injection over the course of five minutes via the auricular vein.
DCE-US Study
For all of the DCE-US studies, we used SonoVue (Bracco, Milano, Italy), which is a second-generation ultrasound contrast agent [13]. In each DCE-US study, a rabbit with a VX2 liver tumor was injected with a 1.0-mL bolus of SonoVue followed by 2.0 mL of a normal saline flush through the auricular vein. Ultrasound examinations were performed using an AplioXG ultrasound scanner (Toshiba Medical Systems, Otawara, Japan) using a 805AT linear probe (6-12 MHz, mean 9 MHz). A fundamental B-mode ultrasound was used to detect the VX2 liver tumors and to measure the longest dimension of the tumor. For the DCE-US imaging, the vascular recognition imaging mode with a low mechanical index of 0.06 was used, as it could detect the signal generated by microbubbles [14]. DCE-US images were continuously recorded beginning at the time of the SonoVue injection and for three additional minutes (four frames per second).
DCE-US Imaging Data Analysis
Quantitative DCE-US parameters were obtained using the dedicated software CHI-Q (Toshiba Medical Systems). By manually drawing a region of interest (ROI) along the margin of the tumor at a selected frame, the ROI would then be auto-positioned throughout all of the study images. If there were changes in the tumor position due to respiratory motion during the exam, we could adjust the ROI at a particular frame and the software would interpolate the ROI positions between two frames of the different ROIs and then automatically retrack the later ROI [14]. From the time-intensity curves of the ROIs, which were generated by analyzing the threeminute raw linear data of the DCE-US imaging, the following perfusion parameters were obtained: peak intensity (PI), slope coefficient of the wash-in, time to peak intensity (TTP), mean transit time (MTT), total area under the time-intensity curve (AUCtotal), AUC during the wash-in (AUCin), and AUC during the wash-out (AUCout).
Histologic Analysis
Immediately after the seventh day of follow-up imaging, all of the rabbits were sacrificed under deep anesthesia induced by an intravenous injection of 5 mL of KCl, after which they were frozen at -70°C. Pathologic specimens were sectioned in the transverse plane at 1-mm intervals and a representative microscopic section was selected that included the longest dimension of the tumor. The necrotic fraction (NF) was defined as the division of the area of tumor necrosis by the area of the total tumor. The NF for each tumor can be calculated by manually drawing regions of interest to circumscribe the outer tumor borders and regions of tumor necrosis on hematoxylin and eosin (H&E)-stained tissue sections using ImageJ analysis software (http://rsb.info.nih.gov/ij). To determine the histological vascular tumor parameters, hot spots, referring to higher vascular density areas than those in the rest of the tissue on the CD31-stained tissue sections, were chosen at low magnification (×40) and CD31-stained vessels were counted at high magnification (×200, 0.544 mm2). The mean of three measurements in the hot spot areas was used as the mean vessel density (MVD) of the tumor.
Statistical Analysis
To assess the reproducibility of the DCE-US parameters, the measured values seen on baseline and the two-hour follow-up in the control group (n=6) were compared and corresponding coefficients of variation (CVs) within the subjects were calculated. CVs of ≤10%, 10%-25%, and ≥25% were considered to be of good, moderate, and poor reproducibility, respectively [15]. In the CKD-516-treated group (n=8), repeated measures analysis of variance (ANOVA) was performed to determine what change in tumor perfusion would be significant at different points in time, and when significant differences were found, Bonferroni-adjusted pairwise comparisons were performed. In order to evaluate whether DCE-US parameters could be used as early predictive indicators of the tumor response, the relative percentage changes in the DCEUS parameters between the baseline and the follow-up checks until 24 hours were correlated with the relative percentage changes in tumor size during seven days using Pearson correlation in the CKD-516-treated group. The histological features of the control and the treated group, including the NF and MVD, were compared using the Student t-test. A P-value less than 0.05 was regarded as statistically significant. All statistical analyses were performed using MedCalc ver. 12.4.0.0 (MedCalc Software, Ostend, Belgium).
Results
Reproducibility of DCE-US Parameters
In the six control group rabbits, the PI, TTP, AUCtotal, AUCin, and AUCout showed moderate reproducibility with CVs of 24.6%, 18.8%, 17.9%, 16.1%, and 20.9%, respectively, while the slope coefficient of the wash-in and MTT showed poor reproducibility with CVs of 47.7% and 34.8%, respectively. Therefore, we used the five DCEUS parameters of moderate reproducibility, including PI, TTP, AUCtotal, AUCin, and AUCout to assess the serial perfusion change of the VX2 liver tumor following administration of CKD-516 in the treated group.
Serial Change in DCE-US Parameters after VDA Treatment
The serially measured DCE-US parameters and relative percentage changes compared to the baseline values are summarized in Table 1. After CKD-516 administration, the tumor perfusion parameters, including the PI, AUCtotal, AUCin, and AUCout, began to decrease from those seen on the initial follow-up (two hours after treatment) by more than 70% and remained decreased until the 24-hour followup. The TTP had increased at the 24-hour follow-up by 46.5% (from 37.0 to 54.2 sec). Repeated measures ANOVA revealed statistically significant differences in the PI, AUCtotal, and AUCout over time (from baseline to the 24-hour follow-up; P<0.05) (Figs. 1, 2). The Bonferroni-adjusted pairwise comparison test revealed that the AUCtotal and AUCin, as seen on the two-hour follow-up, were significantly lower than the baseline values (P<0.05).

Dynamic contrast-enhanced ultrasonography (DCE-US) images obtained in the vascular recognition imaging mode and the corresponding time-intensity curves of a rabbit VX2 liver tumor.
A, B. The DCE-US studies performed (A) before and (B) six hours after CKD-516 treatment revealed that CKD-516 treatment induced marked decrease in tumor vascularization. Note that the scales of the Y-axis of (A) and (B) differ (ranges of 0.02-1.20×10-4 and 0.14-5.50 ×10-5, respectively).
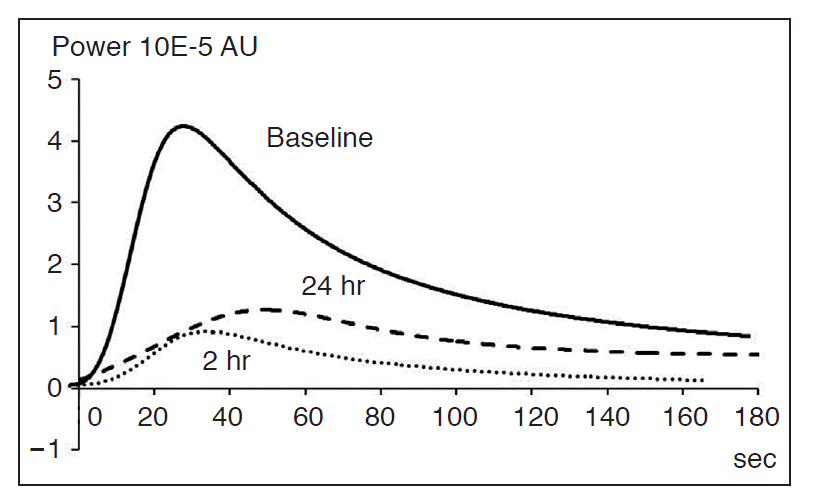
Serial time-intensity curves in one study subject before, two hours after, and 24 hours after CKD-516 administration.
After the CKD-516 treatment, both the peak intensity (PI) and the area under the time-intensity curve (AUC) were markedly decreased at the two-hour follow-up compared to the baseline values (from 4.2 to 0.9 au for the PI; from 326.0 to 59.9 au for the AUCtotal), whereas the time to the peak increased at the 24-hour follow-up (from 22.1 to 46.6 sec).
Early Prediction of Tumor Response to VDA Treatment Using DCE-US Parameters
Among the eight rabbits in the treated group, one died after the 24- hour follow-up. Therefore, the DCE-US parameters of the remaining seven rabbits were evaluated in order to determine the early predictors of tumor response to VDA treatment. The tumor response was assessed in terms of the change in tumor size during the sevenday experimental period. The longest tumor dimension was the mean±SD of 11.0±5.3 mm at baseline and 16.9±5.8 mm seen at the seven-day follow-up. The relative change in tumor size during the experimental period was 63.5%±37.0%, which did not differ significantly from that of the control group (65.4%±39.1%, P>0.05). Pearson correlation test demonstrated no relative percentage changes in the DCE-US parameters until the 24-hour follow-up showed a significant correlation with the relative changes in tumor size in the CKD-516-treated group (P>0.05).
Comparison of the Histological Features of the Control Group and the Treated Group
The NF of the tumor did not differ significantly between the control group (mean±SD of 45.8%±23.2%) and the CKD-516-treated group (36.2%±23.2%, P>0.05). Regarding the MVD, there was no statistically significant difference between that of the control group (19.7±8.1) and the CKD-516-treated group (11.8±4.9, P>0.05) (Fig. 3).

Immunohistochemistry of the VX2 liver tumors showing microvessels at a 7-day follow-up.
On the microscopic specimen (CD31 staining, ×200). A. A tumor in the CKD-516-treated group shows a smaller number of microvessels (arrowheads). B. Another tumor in the control group shows a greater number of CD31-stained microvessels (arrowheads) and more prominent septa (asterisks).
Discussion
Our study results demonstrate that DCE-US was useful for measuring the serial, quantitative changes in the tumor perfusion in a rabbit VX2 liver tumor model after a novel VDA (CKD-516) administration. As a potent VDA, CKD-516 disrupted the tumor perfusion as soon as two hours after administration and thus resulted in a significant decrease in the DCE-US parameters, including the PI, AUCtotal, AUCin, and AUCout, during the time from baseline to the 24-hour follow-up (P<0.05), and pairwise comparison tests revealed that the AUCtotal and AUCin, as seen at the two-hour follow-up, were significantly lower than the baseline values (P<0.05).
In this study using liver tumor models, among the DCE-US parameters, the PI, TTP, AUCtotal, AUCin, and AUCout showed moderate reproducibility with CVs of 10%-25%. As the reproducibility of the measured parameters is one of the most important features of longitudinal studies, those DCE-US parameters can be expected to be valuable imaging biomarkers for monitoring the therapeutic effect of VDAs [16]. When we performed serial follow-up DCE-US at two, four, six, and 24 hours after CKD-516 administration in a rabbit VX2 liver tumor model, a maximal decrease (about-70%) in perfusion parameters including PI, AUCtotal, AUCin, and AUCout was found at the time of the two-hour follow-up. As VDAs induce vascular collapse and destroy pre-existing vessels, VDA effects typically occur within a few hours, while anti-angiogenic drug effects occur after days to weeks [4,17]. Therefore, an early and optimal follow-up imaging schedule is critical for evaluating the therapeutic efficacy of VDA. Until now, there have been only a few studies reporting the imaging timing after VDA treatment. Lavisse et al. [18] reported that that the maximum effect of the VDA, AVE8062, was observed at the six-hour follow-up, which differed from the one-hour or 24-hour follow-ups using DCE-US in a subcutaneous, melanoma-bearing mouse model. In our study, following CKD-516 treatment, the serially measured PI, AUCtotal, and AUCout decreased significantly over time (P<0.05) and post-hoc analysis revealed that the AUCtotal and AUCin were significantly lower at the two-hour follow-up than the baseline values (P<0.05). Although there was no statistical significance (P>0.05), the TTP had increased at the 24-hour follow-up by 46.5%. Our results are in good agreement with previous DCE-US studies evaluating the therapeutic effects of other VDAs, specifically, that AVE8062 induced a marked decrease in the PI and an increase in the TTP as seen at a six-hour followup [18], and 5, 6-dimethylxanthenone-4-acetic acid resulted in a significant decrease in the AUC as seen at a 24-hour follow-up [19]. As VDAs reduce tumor vascularity and the DCE-US contrast agent (SonoVue) is an intravascular tracer, the signal strength generated by microbubbles following VDA treatment should decrease during the entire scanning time, and, therefore, the PI and AUC of the timeintensity curve should decrease [20]. An increase in the TTP indicates slower penetration of the contrast media into the tumor, and a prolonged presence of TTP after VDA treatment would be explained by the reduction of tumor blood vessels, based on the histological findings of previous studies [18,21].
No significant correlation was found between the relative changes in DCE-US parameters at any of the points in time and the relative change in tumor size over the course of the seven days after CKD-516 treatment. In other words, in our study, none of the DCE-US parameters were shown to be early predictors of the tumor response. Several studies have shown that the quantitative parameters derived using functional imaging techniques such as DCE-MR, DCE-US, and [18F] fluorodeoxyglucose positron emission tomography would be helpful for predicting the long-term outcomes of anti-angiogenic drug treatment, whereas only a few previous studies have suggested a useful predictor of tumor response for VDA treatment [4,22-24].
The histological features of the tumor, including the NF and MVD, seen at the time of the seven-day follow-up, showed no statistically significant differences in the control group and the CKD-516-treated group. A previous study which compared the histologic findings of VX2 carcinomas in a rabbit tumor model between control and CKD- 516-treated groups at four-hour and 36-hour follow-up checks, demonstrated that the degree of tumor necrosis was significantly greater in the treated group than in the control group at the time of the 36-hour follow-up [11]. The change in the degree of tumor necrosis at different points in time could be explained by the action mechanism and duration of the VDA, as it rapidly disrupts tumor vessels, thus causing central tumor necrosis. Then the drug effect usually disappears within 1-2 days and tumor regrowth may occur from the periphery of the tumor [3,25]. Regarding the MVD, a previous study also did not show significant difference between the control and the CKD-516-treated groups at four-hour and 36-hour follow-ups, whereas the DCE-MR parameters, similar to our study results with DCE-US, significantly decreased both at four-hour and 36-hour follow-ups after CKD-516 treatment [11]. This discrepancy between the early perfusion change by the VDA and MVD might be explained by that the main cause of the decreasing tumor perfusion within a few hours after VDA treatment may be due to vascular collapse induced by VDA, rather than decrease in MVD [18]. In addition, as the VDA effect would disappear within one or two days and the peripheral tumor usually survives and regrows, the histologic features at the seven-day follow-up might not be expected to differ between the control and treated-groups, just as we found in our study [3,4].
This study has several limitations. First, each DCE-US study was performed using the two-dimensional technique after the operator selected an imaging plane including the longest dimension of the tumor, therefore, the imaging planes of the serial DCE-US might not be identical. A three-dimensional study including the entire tumor volume would be required in order to overcome this limitation [26]. Second, as we only evaluated the histologic features on the sevenday follow-up, any early changes in the DCE-US parameters and corresponding histologic changes could not be correlated.
In conclusion, our results suggest that a novel VDA (CKD-516) treatment causes disruption of tumor perfusion as early as 2 hours after treatment and the early hemodynamic change induced by CKD-516 treatment can be effectively quantified using DCE-US.
Notes
No potential conflict of interest relevant to this article was repoted.
Acknowledgements
This research was partially supported by a grant from Seoul National University Hospital Research Fund (04-2011-0400) and by a grant from the Chong Kun Dang Pharmaceutical Corporation. We would like to thank Bonnie Hami, MA (USA) for her editorial assistance in the preparation of this manuscript.

